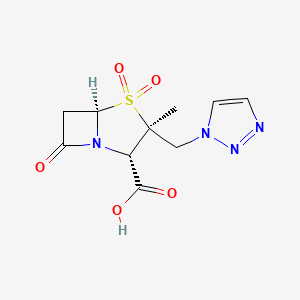
Tazobactam; Tazobactam acid; 89786-04-9; Tazobactamum; YTR-830H; CL-298741
(2S,3S,5R)-3-methyl-4,4,7-trioxo-3-(triazol-1-ylmethyl)-4$l^{6}-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
CAS 89785-84-2 SODIUM SALT
TAIHO Innovator
| MOLECULAR FORMULA: | C10H12N4O5S |
|---|---|
| MOLECULAR WEIGHT: | 300.29108 g/mol |
Tazobactam is a beta Lactamase Inhibitor. The mechanism of action of tazobactam is as a beta Lactamase Inhibitor.
Tazobactam is a penicillanic acid sulfone derivative and beta-lactamase inhibitor with antibacterial activity. Tazobactam contains a beta-lactam ring and irreversibly binds to beta-lactamase at or near its active site. This protects other beta-lactam antibiotics from beta-lactamase catalysis. This drug is used in conjunction with beta-lactamase susceptible penicillins to treat infections caused by beta-lactamase producing organisms.
Tazobactam is a pharmaceutical drug that inhibits the action of bacterial β-lactamases, especially those belonging to the SHV-1 and TEM groups. It is commonly used as its sodium salt, tazobactam sodium.
Tazobactam is combined with the extended spectrum β-lactam antibioticpiperacillin in the drug piperacillin/tazobactam, one of the preferred antibiotic treatments for nosocomial pneumonia caused by Pseudomonas aeruginosa. Tazobactam broadens the spectrum of piperacillin by making it effective against organisms that express β-lactamase and would normally degrade piperacillin.[1]
Tazobactam is a heavily modified penicillin and a sulfone.


PAPER

Synthesis
PATENT
CN 104031065
[2S- (2 α, 2 β, 5 α)] -3- methyl _7_ oxo _3_ (1Η-1,2,3_ triazol-1-ylmethyl) -4- thia-1-azabicyclo – [3,2, O] – heptane-2-carboxylic acid 4,4-dioxide.
The structural formula:
The first from 6-aminopenicillanic acid (6-APA) prepared by starting from the Hall TW et al., Its structure is to add a triazole ring on the basis of sulbactam to improve the effect of inhibiting the enzyme, which is currently lactam best clinical results β_ inhibitor, with high stability, low activity, low toxicity, inhibiting activity and other characteristics. 1992, tazobactam combination drug tazobactam / piperacillin (1: 8) for the first time in France the market, used to treat a variety of bacterial infections.
The literature related to the different synthesis Tazobactam triazole ring according to the introduction, there are two main ways of preparation methods: the azide cycloaddition synthetic triazole five-membered ring and the side chains directly added triazole ring .
Preparation Method One: the azide cycloaddition method, as shown below:
The azide cycloaddition preparation method, which is penicillanic acid diphenylmethyl ester sulfoxide as raw material, open-loop, chloride, azide, oxidation, alkyne cycloaddition, deprotection steps to obtain cilostazol Batan, although each step is quite simple and easy for industrial production, at present most manufacturers use this route, but its route is longer, and there is the azide reaction byproducts generated a large number of six-membered ring, the total yield compared low.
Preparation Method two: direct plus side chains triazole ring
Direct plus side chains triazole ring Preparation mainly disulfide nucleophilic ring was open and IH-1,2,3- triazole occurred directly in acetic acid in the presence of mercury or mercury oxide substituted rings (US4898939) or directly with the IH-1,2,3- triazole silver salt catalyzed reaction of iodine (Synthesis, 2005,3,442-446), as shown below:
And the use of methyl chloride in an alkaline environment and iodine catalyzed substitution to generate the target product (CN200810238479 with 1H-1,2,3- triazole; Shanghai Second Medical University, Shanghai 2009/20 (5): 388- 391), as shown below:
Direct plus side chains triazole ring preparation method because of its short synthetic route, avoiding the risk of high temperature and pressure addition is currently a hot tazobactam drug synthesis research. Since the compound (4) the sulfur atom lone pair of electrons more of a halogen atom (Cl, Br,
I) have a role to leave, under alkaline action by 1H-1,2,3- triazole nucleophilic attack IH ions generated carbocations prone to rearrangement to form a six-membered ring by-products higher probability, if the sulfur atom is oxidized to a sulfone, a sulfur atom, provided no lone pair of electrons, although able to increase its stability, but at the same time a halogen atom (Cl, Br, I) leaving passivation effect, such that the nucleophilic replace hardly occurs while using the expensive raw mercury and silver salts of heavy metals, higher costs, greater environmental pollution, which greatly restricted the industrial scale production.
Synthetic route of the present invention are as follows:
Example 1: Preparation of 3-methyl – [2-oxo-4- (2-benzothiazolyl dithio) -1-azetidinyl] -3-butene diphenylmethyl ester (Compound 3) Preparation of
In penicillanic acid diphenylmethyl ester sulfoxide (compound 2) as a raw material, according to the literature (Synthesis, 2005,3,442-446) preparation, to give a pale yellow crystalline solid from acetone powder at a yield of 95%.
[0019] Examples of 2: 2 β- bromomethyl -2 α- methyl – penicillanic acid diphenylmethyl ester (Compound 4) Preparation of the solid obtained in Example I (Compound 3) 26g (0.05mol) dissolved in 300mL of methylene chloride, cooled to 0 ° C
The following is added 33.5g (0.075mol) of anhydrous copper bromide, after increases in (T5 ° C the reaction was stirred 10-ΐ2 hours, TLC sample testing of raw materials point disappears, and the filter cake was rinsed with 50mL methylene burn, The filtrate was respectively 200mL water, 200mL saturated sodium bicarbonate, 200mL water washing, containing 2β- bromomethyl -2α- methyl – penicillanic acid diphenylmethyl ester (compound 4) in methylene chloride was used directly in the next step reaction.
Examples 3 [0020]: 2 β – bromomethyl -2 α – methyl – penicillanate _1 β _ oxide diphenylmethyl ester (Compound 5) Preparation of
Of Example 2 was 2 β – bromomethyl -2 α – methyl – penicillanic acid diphenylmethyl ester (Compound 4) in dichloromethane was added 30mL of methanol, cooled to -5 ° C or less, dropwise 30mL50 % hydrogen peroxide / sodium tungstate mixture for about 30 minutes after the dripping, and the temperature at (T5 ° C incubation for 4 hours, then heated to 1 (T15 ° C incubation for 4 flying hours, TLC sample testing of raw materials (Compound 4) disappear , was added 200mL 7jC, stirred for five minutes, standing layer, the liquid layer was then washed with dichloromethane material 200mL 5% aqueous sodium bicarbonate to give comprising 2β – bromomethyl -2 α – methyl – di penicillanate phenylmethyl ester -1 β – oxide (Compound 5) in methylene chloride was used directly in the next reaction.
[0021] Example 4: 2 @ – (! 1 1-1,2,3- triazole group) -20- methyl – penicillanic acid diphenylmethyl ester 1 @ – oxide (compound 6) Preparation
Of Example 3 was 2 β – bromomethyl -2 α – methyl – penicillanic acid diphenylmethyl ester -1 β – oxide (Compound 5) in dichloromethane was added 60mL methanol, 30mL water and 10.35g (0.15mol) 1H-1,2,3- triazole, cooled to below 5 ° C, was added 26g anion resin, temperature 5 ~ 10 ° C and stirred overnight (more than 24 hours), samples of raw materials by TLC (Compound 5) disappears, filtered, and the filtrate was added 200mL of water, standing layered material liquid dichloromethane layer was added anhydrous magnesium sulfate and activated carbon decolorization dehydration process, concentrated and dried under reduced pressure, the residue was added 60mL of methanol was dissolved by heating, stirring slowly cooled to (T5 ° C crystallization, precipitation continued until most of the solids after cooling to below -10 ° C for about 4 hours, filtered, and the cake was rinsed with cold methanol and vacuum dried to give a white solid (compound 6) Hg, yield 82% (Compound 3 by meter), mp: V; ESI (m / z):. 450 ,; IHNMR (CDl3) Examples 5: 2β- (1Η-1,2, 3- triazole-yl) -2α- methyl – penicillanic acid diphenylmethyl ester 1,1-dioxide (Compound 7) Preparation of
The 9g (0.02πιο1) 2β – (1H-1,2,3- triazole group) _2 α – methyl – penicillanic acid diphenylmethyl ester 1-oxide (compound 6) was dissolved in 225mL dichloro methane, adding 45mL glacial acetic acid, cooled to below 0 ° C, was added in portions
3.8g (0.024mol) of potassium permanganate. After the addition was completed in 5 ~ 10 ° C incubated overnight (more than 16 hours), sampled by HPLC completion of the reaction, insolubles were removed by filtration, the filtrate was added to 200mL water, stirred for five minutes, allowed to stand The layers were separated and then washed with 200mL saturated aqueous sodium bicarbonate, methylene chloride stock solution layer was dehydrated by adding anhydrous magnesium sulfate and decolorizing charcoal treatment, the remaining concentrated under reduced pressure to about 50mL volume, slowly with stirring to a cooled (TC hereinafter Crystallization 2 hours, filtered, rinsed with a small amount of methylene chloride, dried in vacuo to give a white solid (Compound 7) 8.85g, yield 95%, mp: 201-206 ° C; ESI (m / z): 466, .; IHNMR (CDl3) Example 6: Preparation of tazobactam he (Compound I),
The 1g (0.021mol) 2 β – (1H-1,2,3- triazole group) _2 α – methyl – penicillanate 1,1-diphenyl ester (compound 7) was dissolved in 10mL m-cresol at 50 ~ 55 ° C incubated for 2 hours, cooled to O ~ 5 ° C, was added 200mL of methyl isobutyl ketone, extracted twice with 10mL saturated sodium bicarbonate solution, the combined aqueous layers were dried 10mL ethyl acetate extract miscellaneous twice, and the aqueous layer was added active carbon filtration, and the filtrate was cooled to O ~ 5 ° C, dropping 6mol / L hydrochloric acid to precipitate a solid no longer far, filtered cake was washed with cold water and dried under vacuum to give a white solid tazobactam 5.9g, yield 92%, mp: 136-1380C; ESI (m / z): 300; IHNMR (CDl3) ο
PATENT
WO 2014037893
improved process for the preparation of Tazobactam of formula (I).
(I)
Tazobactam is chemically known as 2a-methyl-2 -(l,2,3-triazol-l-yl)- methylpenam-3a-carboxylate- 1,1 -dioxide and has a very low antibacterial activity. On the other hand, it exhibits a beta-lactamase inhibitory activity when irreversibly bonded to beta-lactamases produced by microorganisms. For this reason, Tazobactam may be used in combination with known antibiotics prone to be inactivated by beta-lactamases to allow them to exhibit their inherent antibacterial activity against beta-lactamase producing microorganisms. Tazobactam as a product is disclosed in US Patent No. 4,562,073.
Considering the importance of Tazobactam there are several literatures available which disclose various processes for the preparation of Tazobactam, some of which are described below.
US patent No. 4,562,073 provides Tazobactam of formula (I) and its derivatives. This patent also describes a process for their preparation as shown in Scheme – 1.
(I )
Scheme – 1
wherein R is hydrogen or trialkylsilyl; R is hydrogen, trialkylsilyl or COOM wherein M is hydrogen, C1-18 alkyl, C2-7 alkoxymethyl, etc., R has the same meaning as M and R represents carboxyl protecting group.
US patents 4,891,369 and 4,933,444 disclose an approach, which involves the preparation of 2a-methyl-2 -triazol lmethylpenam derivative of formula (V)
(V)
wherein R is a carboxy protecting group, by treatment of a β-halomethyl penam derivative of formula (IV), wherein X is chlorine or bromine and R is a carboxy protecting group, with 1,2,3-triazole.
(IV) US patent No. 4,507,239 provides a process which involves the preparation of 2a-methyl-2 -azidomethylpenam derivatives of formula (VII) by treatment of compound of formula (IV) with sodium azide in aqueous aprotic solvents.
In yet another method disclosed in US patent No. 4,895,941, penam sulfoxide of formula,
(II)
wherein R represents a carboxy protecting group, is treated with 2-trimethylsilyl- 1,2,3-triazole in a sealed tube at elevated temperatures to give a mixture which upon column chromatography purification yields 2a-methyl-2 -triazolylmethyl penam derivative of formula (V).
US patent 4,518,533 provides a process for the preparation of intermediate of formula (III)
(HI) wherein the ester of penicillanic acid- 1 -oxide [compound of formula (II)] is reacted with 2-mercaptobenzothiazole in aliphatic hydrocarbon or aromatic hydrocarbon followed by isolation using column chromatographic method.
US patent 7,273,935 provides a process for the preparation of compound of formula (VIII) by reacting compound of formula (III) with cyclising agents like HCl or HBr and sodium nitrite.
(VIII)
wherein R is carboxyl protecting group and L is a leaving group like CI or Br.
US patent 6,936,711 provides a process for the preparation of protected tazobactam [compound of formula (VI)] by reacting compound of formula (VIII) with 1,2,3-triazole using a base.
In addition, US patent namely US 6,660,855, US 7,692,003, and US 7,547,777 claim process for the preparation of crystalline intermediates useful in the preparation of Tazobactam.
In general, de-protection of p-nitrobenzyl/ diphenylmethyl group in penem/penicillin core like Meropenem, Imipenem, Doripenem, Ertapenem, Faropenem, tazobactam and the like utilizes 1-10% of palladium on carbon, like commercially available 1.0%, 2.5%, 5.0%, 7.5% or 10%, which requires high pressure reactor. US patent 4,925,934 provides a de-protection method for 2a-methyl-2 – triazolylmethylpenam derivative of formula (VI) by reaction with m-cresol
(VI)
wherein R is selected from p-methoxybenzyl, diphenylmethyl (benzhydryl), 3,4,5- tirmethoxybenzyl, 2,4-dimethoxybenzyl, 3,5-dimethoxy-4-hydroxybenzyl, 2,4,6- trimethylbenzyl, ditolylmethyl, dianisylmethyl or tert-butyl. The isolated product contains higher amount of m-cresol as an impurity.
US patent 7,674,898 provides a process for the isolation of tazobactam by heating the aqueous solution containing Tazobactam before adjusting the pH. Before adjusting the pH of the aqueous solution containing tazobactam, the said solution was treated with ion-exchange resin column to purify the product. The use of ion-exchange resin and eluting the product is cumbersome on commercial scale.
Considering the importance of Tazobactam in healthcare treatment, the present inventors diligently worked to identify a robust and high yield process for the preparation of Tazobactam having cresol content below 5 ppm. A further purpose of the invention is to provide a manufacturing method that yields Tazobactam and its related intermediates with high purity and productivity.
Scheme:
Preparation of Tazobactam (I)
Into m-cresol was added benzhydryl 3-methyl-7-oxo-3-(lH-l,2,3-triazol-l- ylmethyl)-4-thia-l-azabicyclo[3.2.0]heptane-2-carboxylate 4,4-dioxide (VI) (5 g) and heated at 50-55°C till the completion of the reaction. The reaction mass was diluted with methyl isobutyl ketone. The reaction mass was extracted with sodium bicarbonate solution. The aqueous extract was acidified with hydrochloric acid to pH 3.0-4.0 and washed with methyl isobutyl ketone. Activated carbon was added, stirred and filtered. The filtrate was cooled to 0-5°C, and isopropyl alcohol (20 mL) was added followed by adjusting the pH to 1.0-2.0 using hydrochloric acid. The crystallized product was filtered, washed with water and dried.
Yield: 2.7 g
Purity: 99.9%
m-cresol content: 0.7 ppm
Example 5
Preparation of Tazobactam (I)
Into m-cresol was added benzhydryl 3-methyl-7-oxo-3-(lH-l,2,3-triazol-l- ylmethyl)-4-thia-l-azabicyclo[3.2.0]heptane-2-carboxylate 4,4-dioxide (VI) (5 g) and heated at 70-75 °C till the completion of the reaction. The reaction mass was diluted with dichloromethane. The reaction mass was extracted with potassium carbonate solution. The aqueous extract was acidified with hydrochloric acid to pH 3.0-4.0 and washed with dichloromethane. Activated carbon was added, stirred and filtered. To the filtrate, methanol (20 mL) was added followed by adjusting the pH to 1.0-2.0 using hydrochloric acid at 22-27° C. The crystallized product was filtered, washed with water and dried.
Yield : 2.6g
Purity: 99.9%
m-cresol content : 0.24 ppm
Example 6
Preparation of Tazobactam (I)
Into m-cresol was added benzhydryl 3-methyl-7-oxo-3-(lH-l,2,3-triazol-l- ylmethyl)-4-thia-l-azabicyclo[3.2.0]heptane-2-carboxylate 4,4-dioxide (VI) (5 g) and heated at 60-65 °C till the completion of the reaction. The reaction mass was diluted with dichloromethane. The reaction mass was extracted with potassium carbonate solution. The aqueous extract was acidified with hydrochloric acid to pH 3.0-4.0 and washed with dichloromethane. Activated carbon was added, stirred and filtered. To the filtrate, ethanol (20 mL) was added followed by adjusting the pH to 1.0-2.0 using hydrochloric acid at 22-27° C. The crystallized product was filtered, washed with water and dried.
Yield : 2.6g
Purity: 99.9%
m- ere sol content : 0.31
Reference example- 1
The process disclosed (example-1) in US 4,925,934 was repeated to get Tazobactam
• The above table clearly indicates that the use of water-miscible solvents helps to reduce the m-cresol content to less than 1 ppm.
• The present process obviates the use of ion-exchange resin for the purification (Refer example-1 of US 7,674,898) and provides a robust process for the industrial production of Tazobactam having less than 5ppm, preferably less than lppm. The m-cresol content in tazobactam acid is determined using HPLC with the following parameters
Colum Zorbax SB C8 (150 x 4.6mm, 3.5μ).
Mobile phase Phosphate buffer: Acetonitile
Detector UV at 200 nm
Column temperature 30°C
Flow rate 0.8 mL/min
Run time 15 min.
PATENT
CN 102020663
Example 8:
(I) in a three-necked flask were added CH2Cl2 300mL IOOOmL and 1. 5mol. [1H2SO4 lOOmL, stirring was added 81. 3g (0. 508mol) of bromine was cooled to 0 ° C after, Ilg sixteen burning trimethylammonium ammonium bromide and 35g (0. 508mol) sodium nitrite to the reaction mixture, with continuous stirring, was added portionwise 6-APA 55g (0. 254mol) and dissolved, and stirred at 0~5 ° C Ih, a solution of lmol . L-1 NaHSO3 to K1- starch paper test solution does not change color. And then allowed to stand separated, the aqueous layer was combined organic layer was extracted twice IOOmL CH2Cl2, washed successively with water, 7% aqueous NaHCO3, saturated sodium chloride aqueous solution, to give 6,6-dibromo-containing penicillanic acid in CH2Cl2 solution was used directly in the next reaction.
(2) in IOOOmL three flask, 6,6_-dibromo penicillanic acid in CH2Cl2 solution (about 400ml), cooled to 5 ° C after the addition of benzhydrol 47g (254mmol), DCC (N, N- dicyclohexyl carbodiimide) 52. 3g (254mmol), 1. 8g of concentrated sulfuric acid was added and dissolved with stirring, at 5~10 ° C under stirring for 30min the reaction product was filtered off D⑶ DCC dehydrated to form the (N, N- two cyclohexylurea), liquor spotting, TLC [developing solvent V (cyclohexane): V (ethyl acetate) = 6: 4] to display all the raw materials after completion of the reaction on a rotary evaporator at 30~40 ° C steam dichloromethane, to give the 6,6-dibromo-penicillanic acid diphenylmethyl ester concentrate was used directly in the next reaction.
[0120] (3) obtained in the above Step 6,6-dibromo-penicillanic acid diphenylmethyl ester concentrate was added 500mL three flask, cooled with stirring to (TC, was added 0. 5g cobalt acetate Co (AC) 2 at 0~5 ° C dropping 50mL 30% H202, finished in 30min drip, drip completed at 0~5 ° C thermal reaction, TLC [developing solvent V (cyclohexane): V (ethyl acetate) = 6: 4] track to complete the reaction (about 4h). Still stratification, the organic layer was successively washed with water three times, after 7% NaHC03 was washed twice, the solvent was distilled off under reduced pressure to give 6,6-dibromo-penicillanic alkylene acid diphenylmethyl ester sulfoxide The crude product without purification, was used directly in the next reaction.
(4) the 6,6-dibromo-penicillanic acid diphenylmethyl ester sulfoxide The crude product was dissolved in 4001 ^ tetrahydrofuran, at 101: add 150mL 10% NH4Cl solution, zinc powder was added in four portions 82. 5g (127mol), at intervals IOmin, about 50min addition was completed, plus complete response at 0~10 ° C 30min. Plus zinc filtered through Celite, standing stratified rotating concentrated organic layer recovered tetrahydrofuran. Ethyl acetate was added to dissolve the concentrated solution, washed with water, saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, concentrated under reduced pressure (45 ° C or less) to just precipitate a solid, 0~5 ° C curing crystallization 3h, suction and the filter cake was dried in vacuo to give white crystals of 6,6-dihydro-penicillanic acid sulfoxide, diphenylmethyl ester 70g, 72% yield [6-APA to calculate, yield = weight of dry product / (6-APA was mass X 383)], mp 145 ~147 ° C (literature value of 145 ~148 ° C).
(5) containing 6,6-dihydro-penicillanic acid sulfoxide, diphenylmethyl ester (70g, 0. 182mol), 2- trimethylsilyl-1,2,3-triazole (25. 7g, the 0. 182mol) and toluene (500mL) autoclave purged with nitrogen, then heated to 110~120 ° C, the reaction 4.5h. After cooling, toluene was evaporated, extracted with ethyl acetate (700mL), water (250mL) washed with saturated sodium chloride solution (250mL), dried over anhydrous magnesium sulfate, the solvent was evaporated, and recrystallized from ethanol to give 2a- A yl 23- (1,2,3-triazol-1-yl) methyl penicillanate -3 a- carboxylic acid, diphenylmethyl ester (white solid) 43.48g, 55% yield [yield = dry product Weight / (() • 182X434. 4)], mp 140 ~142 ° C (literature values 141 ~143 ° C).
(6) The 2a- methyl 2 P – (1,2,3`_ triazol _1_ yl) methyl penicillanate _3 a – carboxylic acid diphenylmethyl ester 43. 48g (0.1OOmoI ) was dissolved in 35mL of acetone, was added 70mL of water and 105mL of glacial acetic acid, cooled to 0~5 ° C, was added with stirring a mixture of KMnO4 (23. 7g KMnO4,16. 5g of concentrated phosphoric acid, and 520ml water), with 5mol. L- phosphate, pH was adjusted to 1 6.5, the reaction was stirred at room temperature for 3h. 30% hydrogen peroxide was added dropwise to the reaction solution colorless, filtered, and the resulting crude product was recrystallized from methanol to give 40. 6g as a white solid (2 a- methyl 2 ¢ – (1, 2,3- triazol-1-yl) methylpenicillanate _3 a – carboxylic acid diphenylmethyl ester-dioxide), 87% yield [yield = weight of dry product / (0 100X466.7).]. mp 205~207 ° C (206 ~208 literature values ..).
(7) in 500ml reaction flask was added 2 a- methyl 2 ¢ – (1, 2,3- triazol-1-yl) methyl penicillanate _3 a- two carboxylic acid diphenylmethyl ester oxide 40. 6g (0. 087mol) and 200mL (2mol) between A sprinkle, stirred until solid was completely dissolved, 80 ° C the reaction was kept 4h, cooled to room temperature, was added 600mL of methyl isobutyl ketone, with IOOmL 7% carbonate solution of sodium hydroxide wash, the aqueous layer was separated, the organic layer was washed twice with 150ml, the combined aqueous layer was cooled to 0~5 ° C, with 6mol. L-1 hydrochloric acid to adjust the pH to I~1. 8, white crystals precipitated, pumping filter, 80 ° C drying, dry goods tazobactam 15. 2g, 58% yield [yield = dry goods weight / (0. 087X300. 3)] o mp 136 ~137 ° C (literature value of 136 ~ 138 ° C).
PATENT
- methods of producing β-substituted methyl penam derivatives. For instance, US 4,529,592 discloses a process which involves the treatment of 2α-methyl-2β-azidomethyl penam derivatives of formula (c):wherein R is a carboxy-protecting group, with acetylene, an acetylene derivative or a vinyl derivative under high pressure in a sealed reactor and at elevated temperatures, followed by deprotection with a suitable reagent to get the β-lactamase inhibitor of formula (a).
- The 2α-methyl-2β-azidomethyl penam derivative of formula (c) is in turn prepared from the 2α-methyl-2β-halomethyl penam derivatives of formula (d)wherein R is a carboxy-protecting group and X is chloro or bromo, by treating with sodium azide in aqueous polar aprotic solvents, followed by oxidation.
- US 4,891,369 and US 4,933,444 disclose a different approach, which involves the preparation of 2α-methyl-2β-triazolylmethylpenam derivatives of formula (e) wherein R is a carboxy protecting group and n is 0, by the treatment of a β-halomethyl penam derivative of formula (d), wherein X is chlorine or bromine and R is a carboxy-protecting group, with 1H-1,2,3-triazole.The product obtained can be oxidized and deprotected to get the 2β-substituted methyl penam compound (a).
- US 4,912,213 discloses a reduction method employing lead salts in catalytic amounts to prepare a 2α-methyl-2β-triazolylmethyl penam derivative of formula (e) (n=0-2) from 6-halo or 6,6-dihalo-2α-methyl-2β-triazolylmethyl penam derivatives of formula (f)where X may be Cl, Br, I; Y may be Cl, Br, I or a hydrogen atom; and R is a carboxy-protecting group.
- In yet another method disclosed by US 4,895,941, penam sulfoxide of formula (g), wherein R represents a carboxy-protecting group, is treated with 2-trimethylsilyl-1,2,3-triazole in a sealed tube at elevated temperatures to give a mixture which requires purification by column chromatography to isolate the 2α-methyl-2β-triazolylmethyl penam derivative of formula (e) (n=0).
- As an alternative to the hydrogenation, US 4,925,934 discloses a deblocking method for a 2α-methyl-2β-triazolylmethyl penam derivative of formula (h) by reaction with cresolwhere R is selected from p-methoxybenzyl, 3,4,5-trimethoxybenzyl, 2,4-dimethoxybenzyl, 3,5-dimethoxy-4-hydroxybenzyl, 2,4,6-trimethylbenzyl, diphenylmethyl, ditolylmethyl, dianisylmethyl or tert-butyl.
- [0009]
- In most of the methods involved, 2α-methyl-2β-halomethyl penam of formula (d) is used as the key intermediate. This is true with both the azide/acetylene combo and the triazole route discussed above. However, the 2α-methyl-2β-halomethyl penam of formula (d) itself is an unstable intermediate and therefore manufacturing and storage of this intermediate in large quantities is always cumbersome. This intermediate has been found to degrade on storage even at low temperatures in isolated form as well as in the solution from which it is isolated. Thus, all the operations related to preparation of the intermediate have to be done rapidly, and the isolated intermediate has to be converted to the final product immediately. As a result of these limitations, in-plant scale up always yields by-products which ultimately require purification demands.

Example 1: Preparation of Tazobactam Sodium by route A. (Fig. 1)Step 1.
- 2.5 L of 1.24 molar sulphuric acid (3.125 mol) was stirred at 4°C in a 6 L flask. 218.4 g (1.0 mol) of 6-APA (99%) (compound I) following 601 g (5.05 mol) of potassium bromide and 2000 mL of ethanol were added, maintaining the temperature between 4 to 8°C. Inorganic salts were removed by filtration. The resulting cake was washed by 2 x 1.25 L of cooled dichloromethane. The aqueous phase was extracted twice using the previous washing liquor and 3 x 500 mL of cooled dichloromethane. The organic phases were combined (approx. 4.0 L) and washed with 2 x 200 mL of 30% brine at 4°C. The greenish-brown solution was concentrated to 700 mL in vacuum. The precipitate was removed by filtration and the solution was kept below 0°C and used without further purification in the next reaction step.
Yield: 90% (by titration)
TLC (thin layer chromatography; detection by UV and phosphomolybdic acid, eluent: acetone – methanol 2:1 v/v): Rf 0.65 (BPA), (eluent: acetone – methanol 4:1 v/v) Rf 0.35 (BPA)
- Production of 6α-Bromopenicillanic acid (BPA) (compound II)
Step 2
- 1.8 mol of BPA in 1400 mL of dichloromethane was placed in a 4 L flask. The temperature of the solution was maintained between 0 to 2°C. 2.0 mol peracetic acid in acetic acid solution (342 mL, 40 wt.-% peracetic acid) was added within 100 to 120 minutes, maintaining the temperature of the solution between 0 to 8°C. The color of the solution changed to yellowish-brown. The solution was stirred further 1 hour at 0 to 8°C. The product crystallizes. The slurry was cooled to -10 to -15°C and stirred further 30 minutes then filtered. The cake was washed with 2 x 400 mL of dichloromethane at -10°C. The product was dried at 20 – 25°C in vacuum. The crude product was kept below 0°C and used without further purification immediately (storage time 1 to 2 days) in the next reaction step.
Yield: 314 – 331g (58,9 – 62.1 %) Mp: 130 °C (decomp.)
Cumulative yield of 1st and 2nd steps: 51- 52%
TLC (detection by UV and phosphornolybdic acid, eluent: acetone – methanol 2:1 v/v)
Rf 0.65 (BPA), Rf 0.45 (BPO)
The yield can be improved using higher concentrated peracetic acid.
- . Production of 6α-Bromopenicillanic acid-S-oxide (BPO) (compound III)
Step 3
- In a 4 L flask 272.44 g (0.92 mol) of BPO was dissolved in 120 mL DMF at 25°C. 100.8 g (1,2 mol) of sodium hydrogencarbonate and 229.0 g (1.06 mol) of p-nitrobenzylbromide (PNM) were added portionwise. The slurry was cooled and stirred at 0 to 5°C for one hour. The product was filtered and washed with 2 x 800 mL of cold water. The wet product was placed in a 2 L flask and 1200 mL of methanol was added. The slurry was refluxed for one hour, cooled to -10°C and filtered. The cake was washed with 2 x 800 mL of methanol at -10°C. The product was dried at 25 – 30°C in vacuum and stored at 0°C without further purification in the next reaction step.
Yield: 334.8 g (84.4%) Mp: 130 °C (decomp.)
Cumulative yield of 1st, 2nd and 3rd steps: 46%
TLC (detection by UV, eluent: acetone – methanol 2:1 v/v) Rf 0.75 (BPE), Rf 0.65 (BPO);
(eluent: ethyl acetate – hexane 2:1 v/v) Rf 0.50 (BPE), Rf 0.00 (BPO)
- . Production of 6α-Bromopenicillanic acid-S-oxide p-nitrobenzyl ester (BPE) (compound IV)
Step 4.
- In a 4 L flask 140.84 g (0.826 mol) of 95% 2-mercaptobenzothiazole (MBT) and 345.0 g BPE (0.8 mol) were dissolved in 1360 mL toluene when the solution was heated to 86 – 90°C and an azeotropic mixture of toluene-water was distilled at 450 to 500 mbar. After 3 to four hours, 14 to 16 mL of water was removed using a Dean-Stark apparatus maintaining the temperature between 86 to 90°C. If unreacted BPE could be detected by TLC, a small amount of 2 to 8 g of MBT was added. The solution was refluxed until no starting material could be detected by TLC.
- The solution was evaporated in vacuum between 60 to 70°C. The residual oil was dissolved in 1200 mL of ethyl acetate. After cooling the product crystallizes. The slurry was concentrated in vacuum below 50 °C to 800 mL and 1200 mL isopropyl ether was added to give a well-filterable crystalline slurry that was cooled below 20°C and stirred for additional 24 hours. Subsequently, the product was filtered and washed with 2 x 500 mL cooled isopropyl ether. The product was dried in vacuum between 25 – 30°C.
Yield: 412.8 g (88.9%) Mp.: 116-119°C
Cumulative yield of 1st, 2nd, 3rd and 4th steps: 41%
TLC (detection by UV, eluent: isopropyl ether – ethyl acetate 99:1 v/v) Rr 0.65 (BBE)
- Production of 2-(2-Benzothiazolyldithio)-3-bromo-α-(1-methylethylidene)-4-oxo-1-azetidincacetic acid p-nitrobenzyl ester (BBE) (compound V)
Step 5
- In a 4 L flask 290.24 g (0.5 L) of BBE was dissolved in 1500 mL dichloromethane. The solution was cooled to -2°C. 540 mL of 30% aqueous solution of hydrogen bromide (2.52 mol) was added, keeping the temperature below 0°C. A solution of 103.5 g (1.5 mol) sodium nitrite in 300 mL was added keeping the temperature between 0 to 3 °C. Meanwhile the colour of the organic phase turned to brown. The reaction mixture was stirred about 90 min at 0 to 5 °C until the starting material could not be detected by TLC. 80 g of sodium carbonate (0.75 mol) was added, adjusting the pH to between 6 and 7. The reaction mixture was filtered using perlite as a filter aid. The precipitate was washed with 3 x 100 mL dichloromethane. The combined organic layer was separated and concentrated to 700 mL. The solution was cooled to 20 °C and two litres of isopropyl ether were added slowly. The crystalline suspension was stirred 16 hours at 20 °C and two hours at 0 °C. It was filtered and the product was washed with 2 x 300 mL of cooled isopropyl ether. The product was dried at 20 to 25 °C in vacuum.
Yield: 235.84 g (95.5%) Mp.: 80 °C (decomp.)
Purity: min. 95 %
Cumulative yield of 1st – 5th steps: 39%
The product is sensitive to light and decomposes on silica gel to give cepham.
TLC (detection by UV, eluent: isopropyl ether – ethyl acetate 99:1 v/v) Rf 0.72 (DBPE),
Rf 0.65 (BBE), Rf 0.57 (cepham)
- . Production of 6α-Bromo-2β-bromomethyl-2α-methylpenam-3α carboxylic acid p-nitrobenzyl ester (DBPE) (compound VI)
Step 6
- In a 2 L flask 292.3 g (342 mL, 2.664 mol) trimethylsilylchloride was dissolved in 1300 mL of toluene. 210.1 g (3.20 mol) sodium azide was added and the suspension was stirred and refluxed. The reaction was traced by GC. After 10 to 16 hours less than 0.1% of the starting material could be detected. The suspension was cooled to -5 to 0°C and was filtered (or decanted). The solution (1580 mL) contains 2.40 mol of trimethylsilylazide, which is volatile (Bp: 95°C) and a toxic compound.
- In a 2 L flask 52.63 g (23.7 mL, 0.2 mol) tin(IV) chloride was added to a toluene solution of 2.4 mol of trimethylsilylazide between 20 – 25°C. The solution was stirred 24 hours at 20 – 25 °C while some white precipitate appeared. 197.7 g (0.4 mol) DBPE was added. The suspension was stirred 40 to 70 hours while brown gum appeared. The formation of azide was traced by TLC (eluent isopropyl ether – ethyl acetate 99:1 v/v) Rf 0.72 (DBPE), Rf 0.61 (BAPE), Rf 0.58 (cephambromide) Rf 0.40 (cephamazide).
- Conversion of the starting material to product was less than 50% after 40 hours. Additionally, 0.2 mol of tin (IV) chloride was added, which accelerated the formation of BAPE.
- After no starting material could be detected by TLC, the reaction mixture was quenched with 1200 mL of saturated sodium carbonate solution at 5-10°C. The insoluble material was dissolved by 400 mL ethyl acetate and added to the sodium carbonate solution. The biphasic reaction mixture was stirred 15 minutes, The pH of the lower aqueous phase was between 8 and 9. Perlite (50 g) as a filter aid was added and the suspension was filtered. The cake was washed with 2 x 200 mL of ethyl acetate.
- The combined filtrates were poured into a 5 L separating funnel and the lower aqueous phase was removed and extracted with 2 x 200 mL ethyl acetate. The combined organic phases were washed by 200 mL saturated sodium bicarbonate solution and 200 mL brine. The solvent was removed in vacuum and the residue was suspended in 1000 mL methanol at 0 – 5 °C. The crystalline suspension was stirred 2 to 3 hours at 0 – 5 °C and filtered. The product was washed with 200 mL diisopropyl ether and dried in vacuum at 20 – 25 °C.
Yield: 153.8 g (84.3%)
Purity: 68 ― 70% (by HPLC: mobile phase 0.05 M KH2PO4 – acetonitrile 1:1, pH 6,
Rf 14.33 min)
Cumulative yield of 1st – 6th steps: 33%
- . Production of 6α-Bromo-2β-azidomethyl-2α-methylpenam-3α-carboxylic acid p-nitrobenzyl ester (BTPE) (compound VII)
Step 7
- In a 1 L autoclave 7.6 g (50 mmol) BAPE was dissolved in 640 mL 2-butanone. The solution was cooled down to 0 – 5 °C. The autoclave was pressured three times with nitrogen gas up to six bar. The autoclave was filled with acetylene gas up to 1.5 bar pressure and approx. 36 g acetylene gas was dissolved. The autoclave was heated gradually from 0 °C up to 84 – 94 °C, keeping the pressure between 5 – 6 bar. The reaction mixture was stirred in the autoclave 14 – 20 hours at 84 to 94 °C and pressure of 5 to 6 bar. No starting material was detected by TLC (eluent hexane – ethyl acetate 1:2 v/v) Rf> 0.9 (BAPE), Rf 0.51 (BTPE), Rf0.32 (cephamtriazole).
- The autoclave was cooled down to -20 to -25 °C and 7.6 g BAPE in 50 mL 2-butanone solution was added. The autoclave was heated again to 84 – 94 °C and the reaction mixture was stirred 14 to 20 hours at 84 – 94 °C. The autoclave was cooled and the procedure was repeated with 7.6 g BAPE. The autoclave was cooled down to 20 – 25 °C and opened. The reaction mixture was poured into a 1 L flask and was concentrated in vacuum up to 140 mL. The solution was cooled to 0 – 5°C. The crystalline suspension was stirred for 1 hour and was filtered. The product was washed with 40 mL cool 2-butanone. The product was dried in vacuum at 25 – 30 °C.
Yield: 13.51 g (56.0%) Mp.: 180-182°C (decomp.)
Purity: 98.6% (by HPLC: mobile phase 0.05 M KH2PO4 – acetonitrile 1:1, pH 6,
Rf 8.40 min)
Cumulative yield of 1st– 7th steps: 18%
- . Production of 6α-Bromo-2β-[(1,2,3-triazol-1-yl)methyl]-2α-methylpenam-3α-carboxylic acid p-nitrobenzyl ester (BTPE) (compound VIII)
Step 8
- To a solution of 4.82 g (10.00 mmol) of BTPE in a mixture of 210 ml of acetic acid and 27 ml of water, 3.79 g (23.6 mmol) of KMnO4was added in 30 minutes at room temperature. The progress of the reaction was monitored by TLC. When the reaction was complete, the excess of KMnO4 was destroyed by 30 % H2O2 solution. The reaction mixture was poured into 930 mL of cold water, the precipitated product was filtered and washed with cold water and dried over P2O5, giving compound IX.
Yield: 4,12 g (80 %)
Purity: more than 95 % (HPLC) Mp.: 122-124°C
TLC (detection by UV, eluent: ethyl acetate – hexane 2:1 v/v) Rf0.51 (VIII), Rf 0.23 (IX)
- . Production of p-Nitrobenzyl 6α-bromo-2α-methyl-2β-(1,2,3-triazol-1-yl)methylpenam-3α -carboxylate-1,1-dioxide (compound. IX)
Step 9
- A stainless steel stirred autoclave with a total volume of 1 L was charged with 5.1 g (10 mmol) of compound IX, 2.5 g (30 mmol) of NaHCO3, 1.0 g of 10 % Pd on charcoal, 100 mL of water and 100 mL of ethyl acetate. The autoclave was sealed and flushed with argon, then pressured with hydrogen up to 14 bars. The hydrogenation was carried out at room temperature for 5 h. Completion of the reaction was checked by TLC. The mixture was filtered and the filter washed with water. The aqueous phase was separated, washed with ethyl acetate (2 × 10 mL) and Bu4NNaSO4solution (prepared from 340 mg (1 mmol) of Bu4NHSO4 and 84 mg (1 mmol) of NaHCO3 in 5 mL of water) added. The aqueous solution was extracted with dichloromethane (5 x 10 ml). The combined dichloromethane phases were dried over Na2SO4 and concentrated under reduced pressure to dryness keeping the temperature of the water bath below 20 °C.
Yield: 0.39 g (75 %)
Purity: 95.5 % (HPLC)
HPLC mobile phase: 0.05 M KH2PO4 buffer, pH 2.3
Eluent A: 95 % of 0.05 M KH2PO4 buffer (pH 2.3) plus 5 % acetonitrile
Eluent B: 40 % of 0.05 M KH2PO4 buffer (pH 2.3) plus 60 % acetonitrile
Retention time: 11.53 min
Column: RP-18 endcapped (5µm, 250 mm)
TLC (detection by UV and 1 % AgNO3 in ethanolic solution, eluent: ethyl acetate – hexane 2:1 v/v) Rf 0.23 (IX); (eluent: acetone -methanol 2:1 v/v) Rf 0.48 (Xa)
- . Production of Tetrabutylammonium 2α-methyl-2β-(1,2,3-triazol-1-yl)methylpenam-3α -carboxylate-1,1-dioxide (compound Xa)
Step 10.
- Production of Sodium 2α-methyl-2β-(1,2,3-triazol-1-yl)methylpenam-3α-carboxylate-1,1-dioxide (Tazobactam sodium)
- The residue containing compound Xa (0.40 g) was eluted with water on a column of Amberlite-Na+ cation-exchange resin. The appropriate fractions were concentrated under reduced pressure and finally lyophilized, yielding Tazobactam sodium.
Yield: 0.21 g (85 %)
Purity: 99.5 % (HPLC)
HPLC mobile phase: 0.05 M KH2PO4 buffer, pH 2.3
Eluent A: 95 % of 0.05 M KH2PO4 buffer (pH 2.3) plus 5 % acetonitrile
Eluent B: 40 % of 0.05 M KH2PO4 buffer (pH 2.3) plus 60 % acetonitrile
Retention time: 11.53 min
Column: RP-18 cndcapped (5µm, 250 mm)

PATENT
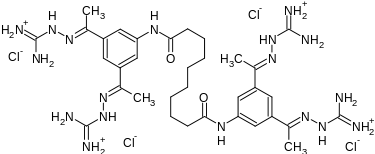
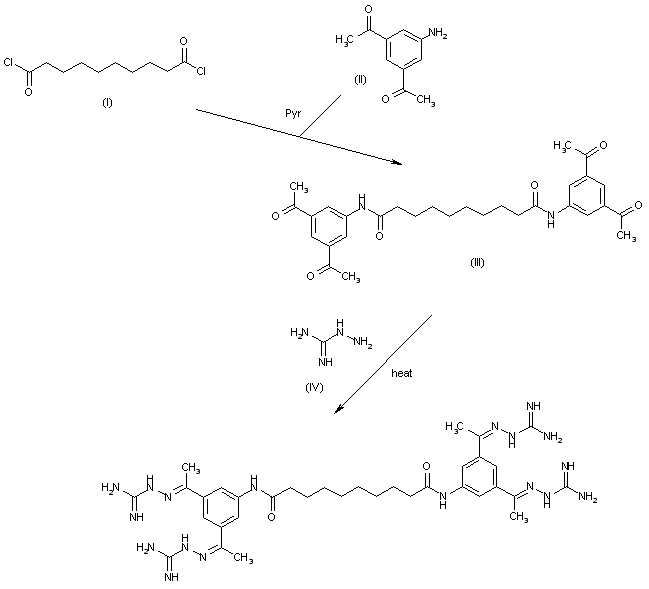
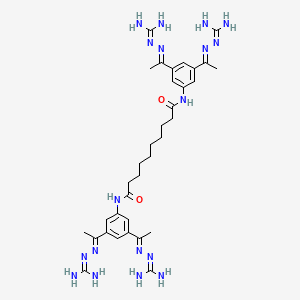
Tazobactam arginine can be a salt consisting of the conjugate base of (2S,3S,5R)-3-((1H-1,2,3-triazol-1-yl)methyl)-3-methyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide (tazobactam) and the conjugate acid of (S)-2-amino-5-guanidinopentanoic acid (L-arginine) in a 1:1 ratio, as represented by the structure below.
References
- Yang Y, Rasmussen BA, Shlaes DM (1999). “Class A beta-lactamases—enzyme-inhibitor interactions and resistance”. Pharmacol Ther. 83: 141–151. doi:10.1016/S0163-7258(99)00027-3.
| CN1037514A | Mar 1, 1989 | Nov 29, 1989 | 大鹏药品工业株式会社 | Process for preparing 2 alpha-methyl-2 beta-(1,2,3-triazole-1-yl) methylpenam-3 alpha-carboxylic acid derivatives |
| US7674898* | Jul 23, 2001 | Mar 9, 2010 | Otsuka Chemical Co., Ltd. | Anhydrous crystal of β-lactam compound and method for preparation thereof |
| REFERENCE | ||
|---|---|---|
| 1 | * | LI YANG ET AL.: ‘Synthesis of Tazobactam, [beta- Lactamase Inhibitor‘ TRANSACTIONS OF TIANJIN UNIVERSITY vol. 8, no. 1, March 2002, pages 33 – 36 |
 | |
 | |
| SYSTEMATIC (IUPAC) NAME | |
|---|---|
| (2S,3S,5R)-3-Methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide | |
| CLINICAL DATA | |
| AHFS/DRUGS.COM | International Drug Names |
| PREGNANCY CATEGORY |
|
| LEGAL STATUS |
|
| ROUTES OF ADMINISTRATION | Intravenous |
| IDENTIFIERS | |
| CAS NUMBER | 89786-04-9 |
| ATC CODE | J01CG02 |
| PUBCHEM | CID: 123630 |
| DRUGBANK | DB01606 |
| CHEMSPIDER | 110216 |
| UNII | SE10G96M8W |
| KEGG | D00660 |
| CHEBI | CHEBI:9421 |
| CHEMBL | CHEMBL404 |
| CHEMICAL DATA | |
| FORMULA | C10H12N4O5S |
| MOLECULAR MASS | 300.289 g/mol |
| PATENT | SUBMITTED | GRANTED |
|---|---|---|
| 2-OXO-1-AZETIDINE SULFONIC ACID DERIVATIVES AS POTENT BETA-LACTAMASE INHIBITORS [EP0979229] | 2000-02-16 | 2002-10-23 |
| DHA-pharmaceutical agent conjugates of taxanes [US7199151] | 2004-09-16 | 2007-04-03 |
| Antimicrobial composition comprising a vinyyl pyrrolidinon derivative and a carbapenem antibiotic or a beta-lactamase inhibitor [EP0911030] | 1999-04-28 | 2005-04-13 |
| 7-alkylidene-3-substituted-3-cephem-4-carboxylates as beta-lactamase inhibitors [US7488724] | 2006-04-06 | 2009-02-10 |
| Sustained release of antiinfectives [US7718189] | 2006-04-06 | 2010-05-18 |
| Conjugate of fine porous particles with polymer molecules and the utilization thereof [US2006159715] | 2006-07-20 | |
| ENGINEERED BACTERIOPHAGES AS ADJUVANTS FOR ANTIMICROBIAL AGENTS AND COMPOSITIONS AND METHODS OF USE THEREOF [US2010322903] | 2009-01-12 | 2010-12-23 |
| Microparticles for the treatment of disease [US2010323019] | 2010-08-19 | 2010-12-23 |
| Packaging System [US2010326868] | 2010-08-30 | 2010-12-30 |
| COMBINATION ANTIBIOTIC AND ANTIBODY THERAPY FOR THE TREATMENT OF PSEUDOMONAS AERUGINOSA INFECTION [US2010272736] | 2010-02-04 | 2010-10-28 |
| CITING PATENT | FILING DATE | PUBLICATION DATE | APPLICANT | TITLE |
|---|---|---|---|---|
| CN102304139A* | Jul 12, 2011 | Jan 4, 2012 | 景德镇市富祥药业有限公司 | Method for preparing 2 beta-methyl penicillanate benzhydryl dioxide |
| CN102304139B | Jul 12, 2011 | Jun 4, 2014 | 江西富祥药业股份有限公司 | Method for preparing 2 beta-methyl penicillanate benzhydryl dioxide |
| CN102382123A* | Mar 10, 2011 | Mar 21, 2012 | 海南美好西林生物制药有限公司 | Preparation method of tazobactam sodium |
| CN102827189A* | Sep 18, 2012 | Dec 19, 2012 | 山东罗欣药业股份有限公司 | Tazobactam sodium compound and pharmaceutical composition thereof |
| US8476425 | Sep 27, 2012 | Jul 2, 2013 | Cubist Pharmaceuticals, Inc. | Tazobactam arginine compositions |
| US8906898 | May 28, 2014 | Dec 9, 2014 | Calixa Therapeutics, Inc. | Solid forms of ceftolozane |
| US8968753 | May 22, 2014 | Mar 3, 2015 | Calixa Therapeutics, Inc. | Ceftolozane-tazobactam pharmaceutical compositions |
| US9044485 | Apr 11, 2014 | Jun 2, 2015 | Calixa Therapeutics, Inc. | Ceftolozane antibiotic compositions |
SEE BACTAM SERIES…………..http://apisynthesisint.blogspot.in/p/bactam-series.html
/////////
O=S2(=O)[C@]([C@@H](N1C(=O)C[C@H]12)C(=O)O)(Cn3nncc3)C
or
CC1(C(N2C(S1(=O)=O)CC2=O)C(=O)O)CN3C=CN=N3
/////////
http://newdrugapprovals.org/2015/12/09/tazobactam/