Tafenoquine…..GSK Launches Phase 3 Malaria Drug Trials « New Drug Approvals:
'via Blog this'
Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, helping millions with websites, 9 million hits on google, 2.5 lakh connections worldwide, P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
Wednesday, 30 April 2014
Monday, 28 April 2014
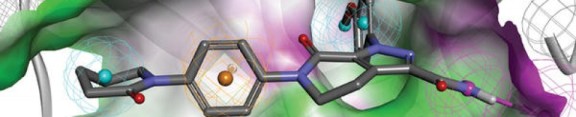
Binimetinib in phase 3 for for the treatment of metastatic or unresectable cutaneous melanoma with NRAS mutations and in combination with LGX-818 in adult patients with BRAF V600 « New Drug Approvals
Friday, 25 April 2014
Thursday, 24 April 2014
Wednesday, 23 April 2014
Tuesday, 22 April 2014
Novel Diacylglycerol Acyltransferase-1 (DGAT-1) Inhibitor..1-(4-(4-Amino-2-methoxy-5-oxo-7,8-dihydropyrido[4,3-d]pyrimidin-6(5H)-yl)phenyl)cyclobutanecarbonitrile « New Drug Approvals
Monday, 21 April 2014
Novel Oxazolidinone Antibacterial Candidate FYL-67 …..(S)-N-((3-(3-Fluoro-4-(4-(pyridin-2-yl)-1H-pyrazol-1-yl)phenyl)-2-oxo-oxazolidin-5-yl)methyl)acetamide « New Drug Approvals
Saturday, 19 April 2014
A novel approach to identify molecular binding to the influenza virus H5N1: screening using molecularly imprinted polymers (MIPs)
In this report we investigate whether a molecularly imprinted polymer (MIP) of an inactivated strain of influenza A H5N1 could be used to help identify molecules capable of binding to, and inhibiting the function of the virus, via either competitive or allosteric mechanisms. Molecules which bind to the virus and induce a conformational change are expected to show reduced binding to the H5N1 specific MIP. Given the importance of molecular recognition in virus replication, such conformational change might also reduce the effectiveness of neuraminidase (N1) for cleaving the sialic groups necessary for virus replication. We show that the method can indeed differentiate between a potent neuraminidase inhibitor, H1 and H5 antibodies, and N1 specific and non-specific monosaccharide substrates. We suggest that such a method could potentially be used in conjunction with traditional biochemical assays to facilitate the identification of molecules functioning via novel modes of action.
Med. Chem. Commun., 2014, Advance Article
DOI: 10.1039/C3MD00272A, Concise Article
DOI: 10.1039/C3MD00272A, Concise Article
Thipvaree Wangchareansak, Arunee Thitithanyanont, Daungmanee Chuakheaw, M. Paul Gleeson, Peter A. Lieberzeit, Chak Sangma
We investigate whether a molecularly imprinted polymer (MIP) of influenza A H5N1 could be used to help identify molecules capable of binding to, and inhibiting the function of the virus, via either competitive or allosteric mechanisms.
We investigate whether a molecularly imprinted polymer (MIP) of influenza A H5N1 could be used to help identify molecules capable of binding to, and inhibiting the function of the virus, via either competitive or allosteric mechanisms.
Friday, 18 April 2014
Wednesday, 16 April 2014
Carbohydrate Derivatives and Glycomimetic Compounds in Established and Investigational Therapies of Type 2 Diabetes Mellitus « New Drug Approvals
Tuesday, 15 April 2014
Monday, 14 April 2014
Friday, 11 April 2014
Ambit Biosciences announces Phase 3 trial comparing quizartinib as monotherapy to chemotherapy regimens in relapsed/refractory acute myeloid leukemia (AML) patients with the FMS-like tyrosine kinase-3 (FLT3)-ITD mutation. « New Drug Approvals
Thursday, 10 April 2014
Cabotegravir, GSK 744 IN PHASE 2 FOR HIV INFECTION « New Drug Approvals
Cabotegravir, GSK 744 IN PHASE 2 FOR HIV INFECTION « New Drug Approvals: "
Cabotegravir, GSK 744,
(3S,11aR)-N-(2,4-Difluorobenzyl)-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydro[1,3]oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide
3S, 1 1 aR)- N-[(2,4-difluorophenyl)methyl]-2,3,5,7, 1 1 , 1 1 a-hexahydro-6-hydroxy-3- methyl-5,7- dioxo-oxazolo[3,2-a]pyrido[1 ,2-d]pyrazine-8-carboxamide"
'via Blog this'
Cabotegravir, GSK 744,
(3S,11aR)-N-(2,4-Difluorobenzyl)-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydro[1,3]oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide
3S, 1 1 aR)- N-[(2,4-difluorophenyl)methyl]-2,3,5,7, 1 1 , 1 1 a-hexahydro-6-hydroxy-3- methyl-5,7- dioxo-oxazolo[3,2-a]pyrido[1 ,2-d]pyrazine-8-carboxamide"
'via Blog this'
Cabotegravir, GSK 744 IN PHASE 2 FOR HIV INFECTION « New Drug Approvals
Cabotegravir, GSK 744 IN PHASE 2 FOR HIV INFECTION « New Drug Approvals: "
Cabotegravir, GSK 744,
(3S,11aR)-N-(2,4-Difluorobenzyl)-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydro[1,3]oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide
3S, 1 1 aR)- N-[(2,4-difluorophenyl)methyl]-2,3,5,7, 1 1 , 1 1 a-hexahydro-6-hydroxy-3- methyl-5,7- dioxo-oxazolo[3,2-a]pyrido[1 ,2-d]pyrazine-8-carboxamide"
'via Blog this'
Cabotegravir, GSK 744,
(3S,11aR)-N-(2,4-Difluorobenzyl)-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydro[1,3]oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide
3S, 1 1 aR)- N-[(2,4-difluorophenyl)methyl]-2,3,5,7, 1 1 , 1 1 a-hexahydro-6-hydroxy-3- methyl-5,7- dioxo-oxazolo[3,2-a]pyrido[1 ,2-d]pyrazine-8-carboxamide"
'via Blog this'
Tuesday, 8 April 2014
Sun Pharma has bought Ranbaxy for $4 billion to create the world’s fifth-biggest generic drugmaker.
 Dilip sanghvi, sun pharma promoter
Dilip sanghvi, sun pharma promoter
The move will make the company the largest pharma firm in India, while Daiichi Sankyo – majority owner of Ranbaxy – will become the second largest shareholder in Sun Pharma with a 9% stake and the right to nominate one director to Sun Pharma’s Board of Directors. http://www.pharmatimes.com/Article/14-04-07/Sun_buys_Ranbaxy_for_4_billion.aspx
Read more at: http://www.pharmatimes.com/Article/14-04-07/Sun_buys_Ranbaxy_for_4_billion.aspx#ixzz2yGIjkMob



Dilip Shanghvi, Managing Director of Sun Pharma said in a release, “Ranbaxy has a significant presence in the Indian pharma market and in the US where it offers a broad portfolio of ANDAs and first-to-file opportunities. In high-growth emerging markets, it provides a strong platform which is highly complementary to Sun Pharma’s strengths,”
Under the agreement, Ranbaxy shareholders will get 0.8 shares of Sun Pharma for each Ranbaxy share.
Arun Sahwney, managing director and chief executive officer of Ranbaxy said in a statement, “Sun Pharma has a proven track record of creating significant long-term shareholder value and successfully integrating acquisitions into its growing portfolio of assets,”
Who Will Benefit?
Daiichi Sankyo Co. Ltd is the parent company of Ranbaxy as they acquired it from previous promoters and investors. As soon as Ranbaxy was acquired, their plants came under a scanner from US Food and Drug Administration (FDA), which troubled Daiichi as their own reputation was under stake.
Now, they will be the most relived entity as Sun Pharma will manage all such cases pertaining to Ranbaxy. Daiichi will now control 9% of Sun Pharma as a result of the current acquisition.
Insiders are claiming that Daiichi will sell this 9% stake as well and come out of the business all together.
Ranbaxy shareholders have cheered this latest development as their shares have gained since the announcement of this deal.
Buserelin a luteinizing hormone-releasing hormone (LHRH) agonist
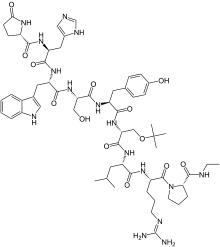
Buserelin
57982-77-1 cas no
(2S)-N-[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2R)-1-[[(2S)-1-[[(2S)-5-(diaminomethylideneamino)-1-[(2S)-2-(ethylcarbamoyl)pyrrolidin-1-yl]-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-[(2-methylpropan-2-yl)oxy]-1-oxopropan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]-5-oxopyrrolidine-2-carboxamide
6-[O-(1,1-dimethylethyl)-D-serine]-9-(N-ethyl-L-prolinamide)-10-deglycinamideluteinizing hormone-releasing factor (pig)
Profact, 57982-77-1, Buserelin (INN), Tiloryth (TN), AC1Q5OOQ, AC1L18ON, D-Ser(Tbu)6EA10LHRH,
Molecular Formula: C60H86N16O13
Molecular Weight: 1239.42424
Therap-Cat: Antineoplastic (hormonal). Gonad-stimulating principle.
Therap-Cat-Vet: Gonad-stimulating principle.
Keywords: Antineoplastic (Hormonal); LH-RH Analogs; Gonad-Stimulating Principle; LH-RH Agonist.

Buserelin is a luteinizing hormone-releasing hormone (LHRH) agonist, a synthetic hormone which stimulates the pituitary gland’s gonadotrophin-releasing hormone receptor (GnRHR). It is used in prostate cancer treatment.
Buserelin stimulates the pituitary gland's gonadotrophin-releasing hormone receptor (GnRHR). Buserelin desensitizes the GnRH receptor, reducing the amount of LH and testosterone. However, there is a concomitant surge in LH and testosterone levels with the decrease in androgens, so antiandrogens must administered.
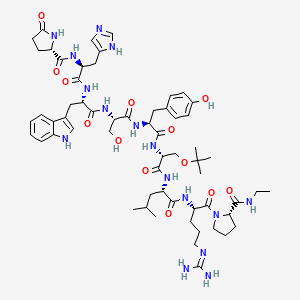 buserelin
buserelin
Properties: [a]D20 -40.4° (c = 1 in dimethylacetamide).
Optical Rotation: [a]D20 -40.4° (c = 1 in dimethylacetamide)
Derivative Type: Monoacetate
CAS : 68630-75-1
Codes: HOE-766
Trademarks: Receptal (Intervet); Suprecur (Sanofi-Aventis); Suprefact (Sanofi-Aventis)
MF: C60H86N16O13.C2H4O2
MW: 1299.48
Percent Composition: C 57.30%, H 6.98%, N 17.25%, O 18.47%
Buserelin is a Gonadotropin-releasing hormone agonist (GnRH agonist). The drug's effects are dependent on the frequency and time course of administration. GnRH is released in a pulsatile fashion in the postpubertal adult. Initial interaction of any GnRH agonist, such as buserelin, with the GnRH receptor induces release of FSH and LH by gonadotrophes. Long-term exposure to constant levels of buserelin, rather than endogenous pulses, leads to downregulation of the GnRH receptors and subsequent suppression of the pituitary release of LH and FSH.
Like other GnRH agonists, buserelin may be used in the treatment of hormone-responsive cancers such as prostate cancer or breast cancer, estrogen-dependent conditions (such as endometriosis or uterine fibroids), and in assisted reproduction.
It is normally delivered via a nasal spray, but is also available as an injection.
Buserelin acetate is marketed by Sanofi-Aventis under the brand name Suprefact and a generic form of Buserelin is now produced by CinnaGen under the brand name CinnaFact.
Buserelin is also marketed under the brand name Metrelef. Metrelef is approved to treat patients with endometriosis by suppression of ovarian hormone production. In ovulation induction Metrelef is used as a pituitary blockade as an adjunct togonadotrophin administration.
Buserelin, a synthetic gonadotropin-releasing hormone (GRH) agonist, specifically binds to GRH receptor presented at anter iorpituitary and increases or decreases the number of receptors in hypophysis through auto- regulation mechanism (G. Tolis et al., Tumor Growth Inhibition in Patients with Prostatic Carcinoma Treated with Luteinizing Hormone-Feleasing Hormone Agonists, Proc. Natl. Acad. Sci. , 79, pl658, 1982).
<5> The synthetic methods for preparing peptides are divided into two methods, i.e., liquid phase synthesis and solid phase synthesis. The liquid phase peptide synthesis of which all the reagents reacts together under the solution phase by being dissolved in the solution, has been reported to show rapid reaction rate however it has disadvantages such as the difficulty in separating and purification of the products. In a while, solid phase peptide synthesis which have been developed based on the theory of R. B. Merrifield, has been reported to have various advantages comparing with the former method for example, convenient to isolation and purification, the 'applicability to automation (Bodanszky et al, In Peptide Synthesis, John Wiley & Sons, 1976). Lots of peptide synthetic resins have been developed to synthesize various peptides after the publication of the theory of R. B. Merrifield till now. For example, chloromethyl polystyrene resin had been developed by Merrifield and Wang resin having 4-alkoxybenzyl alcohol had been developed with modifying the former resin to overcome the disadvantages thereof at the early stage. Various resins to improve the disadvantages of conventional resins have been developed after then and the representative resins among those resins are trityl group introduced 2-chlorotrityl resin and rink amide resin which can provide amide group from the carboxyl terminal of peptide under mild cleavage condition, respectively.
<6> At the early stage, the simple structured type-peptides have been synthesized using by the resins however the complex structured type peptides showing various physiological activities have been synthesized mainly. The peptides comprising unnatural amino acids have been synthesized by chemical synthetic method since the peptides could not be prepared by enzymatic synthesis. Among them, the peptides comprising D-amino acid or aza-amino acid have been reported to have potent physiological activities and further to be developed as a medicine (USP Nos. 6,624,290; 6,069,163; 5,965,538; and 4,634,715). However, the novel method for preparing LH-RH such as goserelin or GnRH peptides using by solid phase synthesis has been still need till now since previously known methods, for example, the methods disclosed in USP No. 5,602,231; EP No. 0518655; USP No. 6,879,289; and USP No. 3,914,412, have been reported to have unsolved problems such as a limit to obtain pure product etc.
http://www.google.com/patents/WO2008044890A1?cl=en
Example 4: Preparation of buserelin
<98> Ig of 2-chlroro trityl chloride resin showing 0.9 mM/g of substitution rate was swollen with 10ml of DMF and the reaction mixture mixed with 768 mg of Fmoc-Arg (N02)-0H (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto to react together. The resulted resin was treated with 20% piperidine to remove the Fmoc residue and the reaction mixture mixed with 615 mg of Fmoc-Leu-OH (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto to react together with a similar way to the above-described method. After washing the resin, the resulted resin was treated with 20% piperidine to remove the Fmoc residue and the reaction mixture mixed with 670 mg of Fmoc-D- SeKtBu)-OH (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto again to react together with a similar way to the above-described method. After washing the resin, the resulted resin was treated with 20% piperidine to remove the Fmoc residue and the reaction mixture mixed with 859 mg of Fmoc-Tyr(OBzI)-OH (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto again to react together with a similar way to the above-described method. After washing the resin, the resulted resin was treated with 20% piperidine to remove the Fmoc residue and the reaction mixture mixed with 726 mg of Fmoc-Ser(OBzI)-OH (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto again to react together with a similar way to the above- described method. After washing the resin, the resulted resin was treated with 20% piperidine to remove the Fmoc residue and the reaction mixture mixed with 742 mg of Fmoc-Trp-0H (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto again to react together with a similar way to the above- described method. After washing the resin, the resulted resin was treated with 20% piperidine to remove the Fmoc residue and the reaction mixture mixed with 1.078g of Fmoc-His(Fmoc)-0H (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto again to react together with a similar way to the above-described method. After washing the resin, the resulted resin was treated with 20% piperidine to remove the Fmoc residue and the reaction mixture mixed with 244 mg of Pyr-OH (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto again to react together with a similar way to the above-described method.
<99> The resin was washed again and 2ml of 1% TFA (Trifluoroacetic acid)/DCM (dichloromethane) per 70mg of peptide resin was added to the resin, eluted to release the peptide from the resin and the elute was collected with 200 microliter of pyridine. The above-described step was repeated five times. The resin was washed with DCM (dichloromethane) and methanol and the elute was collected with the former elute. The elute was concentrated with evaporation and ether was added thereto to obtain the precipitated peptide. The precipitated peptide was performed to coupling reaction with 305 mg of Pro- NH-CH2CH3 (2.4mM) and 303mg of DIC (2.4 niM) in the presence of DCM
(dichloromethane) solvent. The solution was subjected to concentration with evaporator. The resulting concentrate was dissolved in EtOAc, washed with saturated NaHCOs solution, distilled water, 5% citrate solution and dried with anhydrous MgS(V The remaining MgS04 was discarded with filtration and the filtrate was concentrated with evaporation. The benzyl group and Cbz group among the side chain protecting group in the peptide were removed through catalytic hydrogen transfer reaction using by Pd/C and ammonium formate in the presence of methanol. The resulting peptide was purified with reverse phase column chromatography (Shimadzu H-kit, acetonitrile^water= 22:78 → 32:68, 1% increase/min) to isolate pure buserelin (Yield: 40%).
new patent
WO-2014047822
Solid state method for the preparation of buserelin, an LHRH analog useful for the treatment of sexual dysfunction, ovulation, puberty retardation and cancer. Method is under basic conditions and increases yield and purity. This appears to be the first PCT application from Hybio with this target, however several Chinese national filings have been published. Pan, Ma and Yuan are named on several previous solid phase synthesis PCT applications, most recently WO2013117135.
References:
Synthetic nonapeptide agonist analog of LH-RH, q.v. Synthesis: W. Konig et al., DE 2438350; eidem, US4024248 (1976, 1977 both to Hoechst);
A. S. Dutta et al., J. Med. Chem. 21, 1018 (1978).
Clinical pharmacology: A. Lemay et al.,Fertil. Steril. 37, 193 (1982).
Radioimmunoassay in plasma and urine: S. Saito et al., J. Immunol. Methods 79, 173 (1985).
Veterinary use to increase conception rate: K. Moller, E. D. Fielden, N. Z. Vet. J. 29, 214 (1981).
Clinical evaluation in prostatic carcinoma: J. H. Waxman, Br. J. Urol. 55, 737 (1983); as ovulatory stimulant for in vitro fertilization: V. MacLachlan et al., N. Engl. J. Med. 320, 1233 (1989).
Review of pharmacokinetics and clinical profile: R. N. Brogden et al., Drugs 39, 399-437 (1990); of efficacy in prostatic carcinoma: H. J. de Voogt et al., Scand. J. Urol. Nephrol. Suppl 138, 131-136 (1991).
| US5212288 * | Feb 8, 1991 | May 18, 1993 | Syntex (U.S.A.) Inc. | Temporary minimal protection synthesis of serine-containing polypeptides |
| US5510460 * | May 26, 1995 | Apr 23, 1996 | Zeneca Limited | Peptide process |
| US5602231 * | May 26, 1995 | Feb 11, 1997 | Zeneca Limited | Process for making peptides |
| US6028172 * | Feb 10, 1998 | Feb 22, 2000 | Mallinckrodt Inc. | Reactor and method for solid phase peptide synthesis |
| US6897289 * | May 5, 2000 | May 24, 2005 | Lipotec, S.A. | Peptide synthesis procedure in solid phase |
Monday, 7 April 2014
Saturday, 5 April 2014
Dandelion, Burdock, and Cancer
 burdock
burdock
Dandelion root and burdock root are my two most commonly prescribed herbs when chronic conditions require anti-inflammatory, blood purifying alterativ…
 dandelion
dandelion
Dandelion root and burdock root are my two most commonly prescribed herbs when chronic conditions require anti-inflammatory, blood purifying alteratives for gentle detoxification. This includes conditions such as arthritis and cancer. I’ve studied literally hundreds of herbs from around the world, and considering cost, availability, palatability (no small matter, as people with chronic disease like cancer need to be able to take their herbs at least three times a day for months) – there are probably no two more simple and powerful anticancer herbs on the planet than dandelion and burdock.*
After prescribing both of these in strong dose clinically for years with great results (patients feel better, or experience slowing or even complete remission of some cancers), I learned that many professional British medical herbalists also use the same two-herb combination for conditions requiring blood, lymphatic and liver detoxification.
Dandelion, Burdock, and Cancer
 burdock
burdock
Dandelion root and burdock root are my two most commonly prescribed herbs when chronic conditions require anti-inflammatory, blood purifying alterativ…
 dandelion
dandelion
Dandelion root and burdock root are my two most commonly prescribed herbs when chronic conditions require anti-inflammatory, blood purifying alteratives for gentle detoxification. This includes conditions such as arthritis and cancer. I’ve studied literally hundreds of herbs from around the world, and considering cost, availability, palatability (no small matter, as people with chronic disease like cancer need to be able to take their herbs at least three times a day for months) – there are probably no two more simple and powerful anticancer herbs on the planet than dandelion and burdock.*
After prescribing both of these in strong dose clinically for years with great results (patients feel better, or experience slowing or even complete remission of some cancers), I learned that many professional British medical herbalists also use the same two-herb combination for conditions requiring blood, lymphatic and liver detoxification.
Friday, 4 April 2014
ANTHONY CRASTO'S NEW DRUG APPROVALS TOUCHES 2 LAKH VIEWS IN 179 COUNTRIES
ANTHONY CRASTO'S NEW DRUG APPROVALS TOUCHES 2 LAKH VIEWS IN 179 COUNTRIES
DR ANTHONY MELVIN CRASTO Ph.D
WORLDDRUGTRACKER,OTHERS
- Eurekamoments in Organic Chemistry
- NEW DRUG APPROVALS
- WORLD DRUG TRACKER
- Green Chemistry International
- drug regulatory affairs international
- ORGANIC SPECTROSCOPY INTERNATIONAL
- ORGANIC SYNTHESIS INTERNATIONAL
- ALL ABOUT DRUGS
- ORGANIC CHEMISTRY INTERNATIONAL
- Drug Scaleup and Manufacturing International
DR ANTHONY MELVIN CRASTO, Worlddrugtracker, Born in Mumbai in 1964 and graduated from Mumbai University, Completed his PhD from ICT ,1991, Mumbai, India, in Organic chemistry, The thesis topic was Synthesis of Novel Pyrethroid Analogues, Currently he is working with GLENMARK- GENERICS LTD, Research centre as Principal Scientist, Process Research (bulk actives) at Mahape, Navi Mumbai, India. Prior to joining Glenmark, he worked with major multinationals like Hoechst Marion Roussel, now sSanofi, Searle India ltd, now Rpg lifesciences, etc. he is now helping millions, has million hits on google on all organic chemistry websites. His New Drug Approvals, Green Chemistry International, Eurekamoments in organic chemistry are some most read blogs He has hands on experience in initiation and developing novel routes for drug molecules and implementation them on commercial scale over a 25 year tenure, good knowledge of IPM, GMP, Regulatory aspects, he has several international drug patents published worldwide . He gas good proficiency in Technology transfer, Spectroscopy, Stereochemistry, Synthesis, polymorphism etc He suffered a paralytic stroke in dec 2007 and is bound to a wheelchair, this seems to have injected feul in him to help chemists around the world, he is more active than before and is pushing boundaries, he has one lakh connections on all networking sites, He makes himself available to all, contact him on +91 9323115463, amcrasto@gmail.com
Personal Links
- my sites on the net
- DR ANTHONY MELVIN CRASTO
- GOOGLE GROUP ORGANIC PROCESS DEVELOPMENT
- mixxt
- epernicus
- scipeople
- jimdo
- yolasite
- my cv
- slidestaxx
- wordpress blog
- ABOUT ME
- BRANDSITE
- SKILLPAGES
- Academia.edu
- RESEARCHGATE
- DIIGO
- SLIDESHATE
- WIX
- WIX BLOG
- ISSUU
- SCRIBD
- BIZ
- GOOGLE BLOG
- APNACIRCLE
- Eurekamoments in Organic Chemistry
- Organic Chemistry by Dr Anthony
- Green Chemistry International
- Technology Transfer
- Stereochemistry
- Spectroscopy
- Polymorphism
- Reactions in Org Chem
- DR ANTHONY MELVIN CRASTO Ph.D
- Pharmaceuticals
- Medicinal chemistry
- Organic chemistry literature
- Patent related site
- Green chemistry
- Reagents
- R & D
- Molecules
- Heterocyclic chem
- Sourcing
- NEW DRUG APPROVALS
- WORLD DRUG TRACKER
- Green Chemistry International
- drug regulatory affairs international
- ORGANIC SPECTROSCOPY INTERNATIONAL
- ORGANIC SYNTHESIS INTERNATIONAL
- ALL ABOUT DRUGS
- ORGANIC CHEMISTRY INTERNATIONAL
- GOOGLE PLUS
- Drug Scaleup and Manufacturing International
amcrasto@gmail.com
email me if u like my posts
Thursday, 3 April 2014
ATL1102 for MS – Toxicology Study Main Findings
| Sequence Type: DNA fragment | |
| CTGAGTCTGTTTTCCATTCT |
ATL 1102
The antisense oligonucleotide is complementary to a region in the 3'UTR of human ITGA4 (integrin alpha 4) cDNA whose sequence is 5'-CTGAGTCTGTTTTCCATTCT-3'
Phosphorothioate antisense oligonucleotide consisting of a 9-nucleotide central region of deoxynucleotides flanked by 3 2'-O-methoxyethyl (2'-MOE) nucleotides on the 5' end and 8 2'-MOE nucleotides on the 3' end.
Phosphorothioate antisense oligonucleotide consisting of a 9-nucleotide central region of deoxynucleotides flanked by 3 2'-O-methoxyethyl (2'-MOE) nucleotides on the 5' end and 8 2'-MOE nucleotides on the 3' end.
TOORAK, Australia, April 1, 2014 /PRNewswire/ -- Antisense Therapeutics Limited ("ANP" or the "Company") is pleased to advise that results from a chronic toxicity study in monkeys indicate that ATL1102, an antisense oligonucleotide currently under development for the treatment of multiple sclerosis (MS), was well-tolerated when given subcutaneously for a 6-month dosing period at the 2 dose levels tested (1.5 and 3mg/kg/dose). The Company believes that the preclinical and clinical experience to date with ATL1102 should allow dosing in future trials at or above the 1.5 mg/kg/dose level.
read at
http://www.sys-con.com/node/3037721
ATL-1102
ISIS-107248
TV-1102
ISIS-107248
TV-1102
ITGA4 Expression Inhibitors
Signal Transduction Modulators
PHASE 2
Antisense Therapeutics
Isis Pharmaceuticals
Isis Pharmaceuticals
Antisense Therapeutics Limited (ASX: ANP) is an Australian publicly listed biopharmaceutical drug discovery and development company. Its mission is to create, develop and commercialise second generation antisense pharmaceuticals for large unmet markets. ANP has 4 products in its development pipeline that it has in-licensed from Isis Pharmaceuticals Inc., world leaders in antisense drug development and commercialisation - ATL1102 (injection) which has successfully completed a Phase II efficacy and safety trial, significantly reducing the number of brain lesions in patients with multiple sclerosis, ATL1103 a second-generation antisense drug designed to block GHr production and thereby lower blood IGF-I levels and is in clinical development as a potential treatment for growth and other GH-IGF-I disorders, ATL1102 (inhaled) which is at the pre-clinical research stage as a potential treatment for asthma and ATL1101 a second-generation antisense drug at the pre-clinical stage being investigated as a potential treatment for cancer.
ATL1102 is a second generation antisense inhibitor of CD49d, a subunit of VLA-4 (Very Late Antigen-4). In inflammation, white blood cells (leukocytes) move out of the bloodstream into the inflamed tissue, for example, the Central Nervous System (CNS) in MS, and the lung airways in asthma. The inhibition of VLA-4 may prevent white blood cells from entering sites of inflammation, thereby slowing progression of the disease. VLA-4 is a clinically validated target in the treatment of MS. Antisense inhibition of VLA-4 has demonstrated positive effects in a number of animal models of inflammatory disease including MS with the MS animal data having been published in a peer reviewed scientific journal. ATL1102 was previously shown by Antisense Therapeutics to be highly effective in reducing MS lesions in a Phase IIa clinical trial in MS patients.
ATL-1102 is an antisense oligonucleotide in phase II clinical trials at Isis Pharmaceuticals and Antisense Therapeutics for the treatment of relapsing-remitting multiple sclerosis (MS) in a subcutaneous injection formulation. Phase I clinical trials in a subcutaneous injections for stem cell mobilization and preclinical studies of an inhalation formulation of the drug candidate for the treatment of asthma are also being conducted at Antisense Therapeutics.
ATL-1102 is complementary to nt 4288-4207 (3'UTR) of human integrin alpha 4 (ITGA4) cDNA, and thus inhibits ITGA4 expression, blocking the synthesis of CD49d, a subunit of very late antigen-4 (VLA-4). VLA-4 is known to play a part in both the onset and progression of MS, and its inhibition may prevent white blood cells from entering the central nervous system.
ATL-1102 was originally developed at Isis Pharmaceuticals. In December 2001, Isis and Circadian Technologies formed Antisense Therapeutics, established to focus on the discovery and development of antisense therapeutics. As part of the company's formation, Antisense Therapeutics received a license to ATL-1102 and entered into a five-year antisense drug discovery and development program with Isis. In 2008, Antisense licensed ATL-1102 to Teva. In 2010, Teva terminated its licensee agreement with Antisense for the development of ATL-1102 for the treatment of relapsing-remitting multiple sclerosis. The company stated that the compound was not on line with its preferred product pipeline. In 2001, ATL-1102 was licensed to Antisense Therapeutics by Isis Pharmaceuticals. In 2012, development and commercialization rights to the product were licensed to Tianjin International Joint Academy of Biotechnology and
Contact Information:
Website: www.antisense.com.au
Managing Director: Mark Diamond +61 (3) 9827 8999
USA Investor/Media: Joshua Drumm +(1) 212 375 2664;jdrumm@tiberend.com
Australia Investor/Media: Simon Watkin +61 (0)413 153 272;simon@marketconnect.com.au
Website: www.antisense.com.au
Managing Director: Mark Diamond +61 (3) 9827 8999
USA Investor/Media: Joshua Drumm +(1) 212 375 2664;jdrumm@tiberend.com
Australia Investor/Media: Simon Watkin +61 (0)413 153 272;simon@marketconnect.com.au
SOURCE Antisense Therapeutics Limited
Wednesday, 2 April 2014
Tuesday, 1 April 2014
Subscribe to:
Comments (Atom)