Drugmakers will “inevitably” shift from batch to continuous production of tablets and, according to Freeman Technology, a quality by design (QbD) approach is a fundamental requirement.
Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, helping millions with websites, 9 million hits on google, 2.5 lakh connections worldwide, P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
Showing posts with label manufacturing. Show all posts
Showing posts with label manufacturing. Show all posts
Thursday, 9 October 2014
Monday, 2 December 2013
GSK's Cervarix two-dose schedule receives positive opinion from EMA
European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) has issued a positive opinion for the marketing authorisation of a two-dose schedule of GlaxoSmithKline's (GSK) cervical cancer vaccine, Cervarix, in girls aged nine to 14.
Cervarix is designed to prevent infection from HPV types 16 and 18, that cause about 70% of cervical cancer cases. These types also cause most HPV-induced genital and head and neck cancers. Additionally, some cross-reactive protection against virus strains 45 and 31 were shown in clinical trials. Cervarix also contains AS04, a proprietary adjuvantthat has been found to boost the immune system response for a longer period of time.
Cervarix is manufactured by GlaxoSmithKline. An alternative product, from Merck & Co., is known as Gardasil.
The vaccine was developed, in parallel, by researchers at Georgetown University Medical Center, the University of Rochester, theUniversity of Queensland in Australia, and the U.S. National Cancer Institute.
Clinical Trials
Phase III trials have been conducted, including over 18,000 women from 14 countries in Pacific Asia, Europe, Latin America and North America.
As of 2009 the manufacturer was conducting a trial to compare the immunogenicity and safety of Cervarix with Gardasil. Subsequent studies showed Cervarix generated higher antibody levels than Gardasil, the other commercally available HPV vaccine, upon testing seven months later, with twice the level for HPV type 16 and six times for HPV type 18.In addition Cervarix induced twice as many memory B cells as Gardasil for both these HPV strains.
| Vaccine description | |
|---|---|
| Target disease | human papillomavirus (Types 16 and 18) |
| Type | Protein subunit |
Thursday, 24 October 2013
Why Antibody Lots Differ Significantly
http://www.dddmag.com/news/2013/10/why-antibody-lots-differ-significantly?et_cid=3555713&et_rid=523035093&type=cta
fig is Schematic representation of pure and contaminated antibodies carrying histones or histones complexed with DNA. (Source: Biochemistry and Cell Biology)
fig is Schematic representation of pure and contaminated antibodies carrying histones or histones complexed with DNA. (Source: Biochemistry and Cell Biology)
Tuesday, 30 July 2013
Garlic an antifungal, garlic can also support your immune system, reduce cholesterol, and help control blood sugar levels.

Garlic is a proven antifungal . Research studies have shown that garlic is effective against pathogens like Candida. In addition to its use as an antifungal, garlic can support your immune system, reduce cholesterol, and help control blood sugar levels.
You can start taking garlic supplements once you have finished your cleanse and moved on to the strict anti-Candida diet. As always, it is better to take two or three antifungals at once to prevent the Candida from adapting, so you can use garlic in addition to other natural antifungals.
How does Garlic help with Candida overgrowth?
There is a wide range of scientific literature supporting the use of garlic as an antifungal. Much of this research is focused on Candida and similar pathogenic organisms. For example, a 1988 study (see the full text here) found that “the growth of Candida Albicans was found to be markedly inhibited by AGE [aqueous garlic extract]”. According to another article by Huntington College of Health Sciences, “Research has clearly shown that garlic has anticandidal activity, inhibiting both the growth and function of Candida Albicans”.
One of the key compounds in garlic is ajoene, a proven antifungal that has been shown to be effective against many fungal strains. Ajoene is formed from a compound named allicin and an enzyme named allinase. When these two natural compounds come into contact (by chopping the garlic, crushing it or by other means), they form an antibacterial agent named allicin, which then combines to form ajoene. Although this has proven antifungal properties, the exact mechanism by which this happens is not clear. As with other antifungals, scientists suspect that it works by disrupting the cells walls of the Candida yeast cells.
A major advantage of garlic is that it is so easy to include in your treatment plan. Garlic tablets, softgels and oils are widely available, and fresh garlic cloves make a tasty addition to many recipes. You can use garlic as a complement to your other antifungals without having to spend a great deal of money. To get the best results and prevent the Candida yeast from adapting to the treatment, it is best to take two or three antifungals at the same time.
How do you take Garlic?
Garlic products can be found in a number of different forms, in both your supermarket and your health food store. In your supermarket you will find items like fresh garlic cloves, garlic paste, crushed garlic, garlic flakes or garlic powder. Your health food store should stock garlic tablets and garlic oil.
Each type contains different levels of the active ingredients, so make sure to read the ingredients. Here is a basic run-down of the recommended dosage for each type:
- Garlic cloves: 2 to 4 grams per day of fresh, minced garlic clove
- Garlic Tablets: 600 to 900 mg daily, freeze-dried garlic standardized to 1.3% alliin or 0.6% allicin
- Garlic Oil: 0.03 to 0.12 mL three times a day
Who should not take Garlic?
Although a natural remedy, concentrated garlic can still interact with other medicines, so always consult a health professional. Garlic has a blood-thinning property that can be very useful, but can also be dangerous to sufferers of hemophilia or platelet disorders, as well as pregnant women or patients about to undergo surgery.
Side effects from garlic include upset stomach, bloating, bad breath, body odor, and a stinging sensation on the skin from handling too much fresh or dried garlic. Handling garlic may also cause the appearance of skin lesions.
Other side effects that have been reported by those taking garlic supplements include headache, fatigue, loss of appetite, muscle aches, dizziness described as vertigo (namely, the room spinning), and allergies such as an asthmatic reaction or contact dermatitis (skin rash).
Some people may suffer a mild allergic reaction to concentrated garlic. Others may have an upset stomach, body odor, bad breath, headache, loss of appetite or fatigue. It may prompt a skin reaction, such as a stinging in the hands.
Sunday, 28 July 2013
Tuesday, 23 July 2013
New therapy for pancreatic cancer: Phase III clinical trial currently recruiting Australian patients

Currently there is a clinical trial that is recruiting patients from around the globe including sites across Australia. The trial is testing MM-398; a therapy that uses the latest in nanotechnology to deliver the chemotherapeutic agent irinotecan encased in a liposome to cancer patients.1 In particular this trial, named NAPOLI-1 (NAnoliPOsomaL Irinotecan) is recruiting patients with pancreatic cancer who have previously been treated with the chemotherapy agent gemcitabine unsuccessfully i.e. their disease has gone on to spread/progress despite this treatment.2,3
read all here
http://www.virtualmedicalcentre.com/news/new-therapy-for-pancreatic-cancer-phase-iii-clinical-trial-currently-recruiting-australian-patients/18708
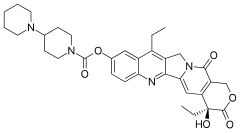
irinotecan
Irinotecan (Camptosar, Pfizer; Campto, Yakult Honsha) is a drug used for the treatment of cancer.
Irinotecan prevents DNA from unwinding by inhibition of topoisomerase 1. In chemical terms, it is a semisynthetic analogue of the natural alkaloid camptothecin.
Its main use is in colon cancer, in particular, in combination with other chemotherapy agents. This includes the regimen FOLFIRI, which consists of infusional 5-fluorouracil,leucovorin, and irinotecan.
Irinotecan received accelerated approval by the U.S. Food and Drug Administration (FDA) in 1996 and full approval in 1998. During development, it was known as CPT-11.
Irinotecan is activated by hydrolysis to SN-38, an inhibitor of topoisomerase I. This is then inactivated by glucuronidation by uridine diphosphate glucoronosyltransferase 1A1 (UGT1A1). The inhibition of topoisomerase I by the active metabolite SN-38 eventually leads to inhibition of both DNA replication and transcription.

Merrimack currently has six oncology therapeutics in clinical development, multiple product candidates in preclinical development and an active Systems Biology-driven discovery effort. MM-398
(Nanotherapeutic)
- Indication:
- Description:
- Target
- Pancreatic Cancer (2nd line, 2 indications), Colorectal Cancer, Glioma
- Nanotherapeutic
- Encapsulated irinotecan
MM-398 is a nanotherapeutic consisting of the chemotherapuetic irinotecan, encapsulated in a liposomal sphere. MM-398 is designed to rely on the natural blood flow of the tumor to direct the therapy directly to the site of the cancer and minimize exposure to non-target cells.
MM-398 in the Clinic
MM-398 is being evaluated in clinical trials for its ability to treat tumors resistant to chemotherapy across multiple types of cancers, including pancreatic, lung, colorectal and glioma. The FDA and the European Medicines Agency granted MM-398 orphan drug designation in 2011 for the treatment of patients with metastatic pancreatic cancer who have previously failed treatment with the chemotherapy drug gemcitabine. Our Phase 3 study, NAPOLI-1 (NAnoliPOsomaL Irinotecan), is currently underway.
posters
http://merrimackpharma.com/library/research/mm-398-preclinical-posters
posters
http://merrimackpharma.com/library/research/mm-398-preclinical-posters

Friday, 19 July 2013
Alkermes Adds Multiple Sclerosis Pill To Expanding Pipeline
Alkermes Adds Multiple Sclerosis Pill To Expanding PipelineTheStreet.comThe company expects to seek permission from FDA to begin human testing of its MMF prodrugs for multiple sclerosis in 2014, with a phase I study slated to begin mid-year. Composition of matter patents have been filed, which if granted, would give the ...
read all at
http://www.thestreet.com/story/11981784/1/alkermes-adds-multiple-sclerosis-pill-to-expanding-pipeline.html?cm_ven=GOOGLEN
read all at
http://www.thestreet.com/story/11981784/1/alkermes-adds-multiple-sclerosis-pill-to-expanding-pipeline.html?cm_ven=GOOGLEN
Santarus Completes Patient Enrollment in CONTRIBUTE Study with UCERIS (budesonide) as Add-on Treatment to 5-ASA Drugs
Santarus Completes Patient Enrollment in CONTRIBUTE Study with UCERIS ...MarketWatchA Biologics License Application for RUCONEST for the treatment of acute angioedema attacks in patients with hereditary angioedema is under review by the U.S. Food and Drug Administration with a response expected in April 2014. Santarus is also ...
read all at
http://www.marketwatch.com/story/santarus-completes-patient-enrollment-in-contribute-study-with-uceris-budesonide-as-add-on-treatment-to-5-asa-drugs-2013-07-18
SAN DIEGO, Jul 18, 2013 (BUSINESS WIRE) -- Santarus, Inc. /quotes/zigman/91852/quotes/nls/snts SNTS -0.61% today announced the completion of enrollment in the CONTRIBUTE clinical study designed to evaluate the incremental benefit of adding UCERIS(R) (budesonide) extended release 9 mg tablets to oral aminosalicylate (5-ASA) therapy for the induction of clinical remission in adult patients with active, mild to moderate ulcerative colitis. UCERIS is currently approved in the U.S. for the induction of remission in patients with active, mild to moderate ulcerative colitis.
read all at
http://www.marketwatch.com/story/santarus-completes-patient-enrollment-in-contribute-study-with-uceris-budesonide-as-add-on-treatment-to-5-asa-drugs-2013-07-18
SAN DIEGO, Jul 18, 2013 (BUSINESS WIRE) -- Santarus, Inc. /quotes/zigman/91852/quotes/nls/snts SNTS -0.61% today announced the completion of enrollment in the CONTRIBUTE clinical study designed to evaluate the incremental benefit of adding UCERIS(R) (budesonide) extended release 9 mg tablets to oral aminosalicylate (5-ASA) therapy for the induction of clinical remission in adult patients with active, mild to moderate ulcerative colitis. UCERIS is currently approved in the U.S. for the induction of remission in patients with active, mild to moderate ulcerative colitis.
Tuesday, 16 July 2013
Top Selling Schizophrenia Drug Abilify (Aripiprazole)
Aripiprazole (brand names: Abilify, Aripiprex, OPC-14597,) is an atypical antipsychotic drug with the chemical name 7-[4-(2,3-dichlorophenyl)-1-piperazinyl]-butyloxy]-3,4-dihydro-2(1H)-quinolinone. It was first discovered by the Otsuka Pharmaceutical Company, Ltd., based in Japan, and in collaboration with Bristol-Myers Squibb began to market the drug in the United States following the approval by the Food and Drug Administration for schizophrenia in November 2002. According to IMS Health, Abilify was the fourth top-selling drug in the United States in 2011, with sales of $5.2 billion.
Chemical Structure of Aripiprazole (brand names: Abilify)
Chemical Synthesis of Aripiprazole(active ingredient for Abilify)
Experimental Procedures for the preparation of Aripiprazole (Abilify)
US 5,006,528 discloses process for the preparation of Aripiprazole in two steps. The first step comprises synthesis of 7-(4-bromobutoxy)-3,4-dihydrocarbostyril (7-BBQ) by alkylating the hydroxy group of 7-hydroxy-3,4-dihydrocarbostyril (7-HQ) with 1 ,4-dibromobutane using potassium carbonate in water at reflux temperature for 3 hours to obtain 7-BBQ in 68% yield. The resulting 7-BBQ is further reacted with 1- (2,3-dichlorophenyl)-piperazine to obtain Aripiprazole.
Preparation of 7-(4-bromobutoxy)-3,4-dihydro-2(1H)quinolinone (7-(4-bromobutoxy)-3,4-dihydrocarbostyril; 7-BBQ)
7-Hydroxy-3,4-dihydro-2(1H)-quinolinone (aka 7-Hydroxy-3,4-dihydrocarbostyril, 60gm) and potassium carbonate (76.3 gm) were taken in acetonitrile (1200ml) at room temperature. To this tetra butyl ammonium iodide (13.7 gm) and 1 ,4-dibromobutane (238.5gm) were added and heated at 40- 45°C for 24 hours. Reaction mass was cooled upto room temperature and was filtered off. The resulting filtrate was distilled off under vacuum. The resultant mass was cooled to 25-30°C and cyclohexane (300 ml) was added under stirring. The resulting solid was filtered off and was dried. The resulting solid was taken in water and was stirred for few minutes. The solid was filtered and dried under vacuum at 55-60°C for 20 hours to obtain title compound. mp 110.5-111 °C; 1H NMR (DMSO-d6) ä 1.81 (2H, m, -CH2-), 1.95 (2H, m, -CH2-), 2.41 (2H, t, J ) 7 Hz, -CH2CO-), 2.78 (2H, t, J) 7 Hz, -CH2-C-CO-), 3.60 (2H, t, J ) 6 Hz, -CH2Br), 3.93 (2H,t, J ) 6 Hz, O-CH2-), 6.43 (1H, d, J ) 2.5 Hz), 6.49 (1H, dd, J) 2.5, 8 Hz), 7.04 (1H, d, J ) 8 Hz), 9.98 (1H, s, NHCO). Anal. (C13H16NO2Br) C, H, N.
Yield: 73-75%; Purity: 93-95%
Preparation of Aripiprazole (7-{4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy}-3,4-dihydroquinolin-2(1H)-one)
7-(4-Bromobutoxy)-l ,2,3,4-tetrahydroquinolin-2-one (50 gm) was taken in acetonitrile (500 ml) at 25-30°C. To this potassium carbonate (67.2 gm) and l-(2,3- dichlorophenyl) piperazine hydrochloride (44.9gm) were added under stirring. The reaction mixture was refluxed at 80-85°C for 8 hours. The reaction mass was cooled to room temperature, filtered and the resulting solid was washed with acetonitrile. To the resulting solid, water was added and was stirred. The solid was filtered off, washed with water and dried under vacuum at 75-80°C for 15 hrs. The resulting crude aripiprazole was crystallized from isopropyl alcohol and water to obtain title compound. Yield: 75-80%; Dimer Impurity: <0.1%. 1H NMR: DMSO-d6 d 9.96 [1H, s, NH]; 7.29 [2H, m, Ar]; 7.13 [1H, q, Ar]; 7.04 [1H, d, Ar]; 6.49 [1H, dd, Ar]; 6.45 [1H, d, Ar]; 3.92 [2H, t, -CH2-O-]; 2.97 [4H, bb,2(-CH2-)]; 2.78 [2H, t, -CH2-N2-)]; 2.39 [4H, m, 2(-CH2-)]; 1.73 [2H, m, - CH2-]; 1.58 [2H, m, -CH2-]. IR:cm-1 3193; 2939; 2804; 1680; 1627; 1579; 1520; 1449; 1375; 1270; 1245; 1192; 1169; 1045; 965; 649; 869; 780; 712; 588.
Preparation of aripiprazole anhydrous Type I using isopropyl alcohol and water
Crude aripiprazole (30 g) was taken in isopropyl alcohol (600 ml) and was heated to 80-85°C. Water (90 ml) was added at the same temperature. Activated carbon was added and the mixture was stirred for 30 minutes at the same temperature. The resulting hot solution was filtered and the bed was washed with hot isopropyl alcohol. The resulting filtrate was cooled to 25-30°C for 4 hours. The resulting solid was filtered, washed with isopropyl alcohol and dried under suction for 1 hour. The resulting wet solid was dried in preheated oven maintained at 100-105°C for 6 hours to obtain title compound.
Yield: 87-89% HPLC Purity: 99.89
Anhydrous crystal D: Below detectable limit (BDL) at limit of detection 1%.
Hydrate A: Below detectable limit (BDL) at limit of detection 1 %.
Particle size distribution: d10=15.83 m, d50=60.12 m, d90=144.99 m
Preparation of aripiprazole anhydrous Type I using ethanol and water
Crude aripiprazole (15 g) was taken in ethanol (300 ml) and water (45 ml) and was heated to 80-85°C for 1-2 hours. The resulting mixture was cooled to 25-30°C within 4 hours and stirred for 3 hours. The resulting solid was filtered and dried under suction for 1 hour. The resulting wet solid was dried in preheated oven maintained at 100-105°C for 3 hours to obtain title compound. Yield: 90% HPLC Purity: 99.9%
Anhydrous crystal D: Below detectable limit (BDL) at limit of detection 1%.
Hydrate A: Below detectable limit (BDL) at limit of detection 1%.
Particle size distribution: d10=22.01 m, d50=105.10 m, d90=232.97 m
References For the Process of Aripiprazole (Abilify,)
Yasuo Oshiro, Seiji Sato, Nobuyuki Kurahashi, Tatsuyoshi Tanaka, Tetsuro Kikuchi, Katsura Tottori, Yasufumi Uwahodo, and Takao Nishi; Novel Antipsychotic Agents with Dopamine Autoreceptor Agonist Properties: Synthesis and Pharmacology of 7-[4-(4-Phenyl-1-piperazinyl)butoxy]- 3,4-dihydro-2(1H)-quinolinone Derivatives; J. Med. Chem. 1998, 41, 658-667
Yasuo Oshiro, Seiji Sato, Nobuyuki Kurahashi; Carbostyril derivatives, Otsuka Pharmaceutical Co., Ltd; US patent 5006528;Issue date: Apr 9, 1991
BANDO, Takuji, YANO, Katsuhiko, FUKANA, Makoto, AOKI, Satoshi ;Method for producing fine particles of aripiprazole anhydride crystals b; OTSUKA PHARMACEUTICAL CO., LTD., WO 2013002420 A1
ZHENG Siji, LIU Xiaoyi, FU Linyong, TAN Bo, ZHOU Min: ARIPIPRAZOLE MEDICAMENT FORMULATION AND PREPARATION METHOD THEREFOR. / FORMULATION DE MÉDICAMENT ARIPIPRAZOLE ET SON PROCÉDÉ DE PRÉPARATION. / SHANGHAI ZHONGXI PHARMACEUTICAL January 2013: WO 2013/000391
CN 201210235157
CN102846543A
CN102850268A;
CN101781246A
GUPTA, Vijay Shankar, KUMAR, Pramod, VIR, Dharam ;Process for producing aripiprazole in anhydrous type i crystals;JUBILANT LIFE SCIENCES LIMITED;WO 2012131451 A1
SRIVASTAVA JAYANT GUPTA Vijay Shankar;Improved process for the preparation of 7.(4-bromobutoxy) 3,4-dihydrocarbostyril, a precursor of aripiprazole;wo2011030213 A1
No Generic Abilify in the US until April 2015
On May 7, 2012, The U.S. Court of Appeals for the Federal Circuit ruled in favor of Otsuka Pharmaceutical Co., Ltd. in its patent litigation against several companies including Israel-based Teva and Weston, Ontario-based Apotex seeking FDA approval to market generic copies of Abilify®. (see the pdf file for the decision upholding the Otsuka patent here). The Federal Circuit affirmed a decision of the U.S. District Court for the District of New Jersey holding that the asserted claims of U.S. Patent No. 5,006,528 (pdf file here) covering aripiprazole, the active ingredient in Abilify®, are valid, thus maintaining patent and regulatory protection for Abilify® in the U.S. until at least April 20, 2015. The case is Otsuka Pharma Co. v. Sandoz Inc., 2011-1126 and 2011-1127, U.S. Court of Appeals for the Federal Circuit (Washington). The lower court case is Otsuka Pharmaceutical Co. v. Sandoz Inc., 07cv1000, U.S. District Court for the District of New Jersey (Trenton).
Chemical Name for Aripiprazole(active ingredient for Abilify): 7-{4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy}-3,4-dihydroquinolin-2(1H)-one
CAS number 129722-12-9
CAS number 129722-12-9
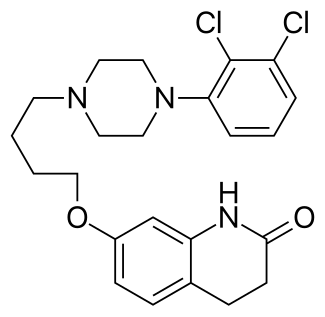
Aripiprazole brand names: Abilify, Aripiprex) is a partial dopamine agonist of the second generation class of atypical antipsychotics with additional antidepressant properties that is primarily used in the treatment ofschizophrenia, bipolar disorder, major depressive disorder, and irritability associated with autism.[1] It was approved by the U.S.Food and Drug Administration (FDA) for schizophrenia on November 15, 2002 and the European Medicines Agency on 4 June 2004; for acute manic and mixed episodes associated with bipolar disorder on October 1, 2004; as an adjunct for major depressive disorder on November 20, 2007;[2] and to treat irritability in children with autism on 20 November 2009.[3] Aripiprazole was developed by Otsuka in Japan, and in the United States, Otsuka America markets it jointly with Bristol-Myers Squibb.
Aripiprazole can be synthesized beginning with a dichloroaniline and bis(2-chloroethyl)amine:
Subscribe to:
Comments (Atom)




