 Cloretazine, Onrigin, VNP40101M, VNP-40101M, 101M, VNP 40101M, Laromustine [USAN], 173424-77-6 casno
Cloretazine, Onrigin, VNP40101M, VNP-40101M, 101M, VNP 40101M, Laromustine [USAN], 173424-77-6 casno
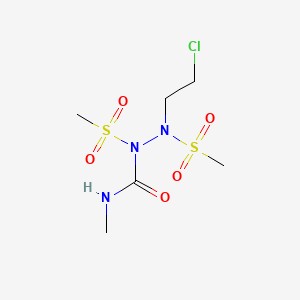
108736-35-22, (2-chloroethyl)-N-methyl-1,2-bis(methylsulfonyl)hydrazinecarboxamide
Molecular Formula: C6H14ClN3O5S2 Molecular Weight: 307.77546
 Vion Pharmaceuticals, Inc. was a New Haven, Connecticut based pharmaceutical company founded in March 1992 to commercialize several discoveries made in the biomedical laboratories at Yale University.
Vion Pharmaceuticals, Inc. was a New Haven, Connecticut based pharmaceutical company founded in March 1992 to commercialize several discoveries made in the biomedical laboratories at Yale University.

Vion Pharmaceuticals, Inc. was a New Haven, Connecticut based pharmaceutical company founded in March 1992 to commercialize several discoveries made in the biomedical laboratories at Yale University.
Two anticancer agents, Onrigin (laromustine), formerly cloretazine (VNP40101M), and Triapine, a ribunucloetide reductase inhibitor similar tohydroxyurea, were in human clinical trials. Onrigin (laromustine), a novel alkylating agent, was evaluated in a Phase 2 trial in elderly de novo poor-riskacute myeloid leukemia (AML). In addition, several trials of Onrigin (laromustine) were conducted in elderly patients with AML and myelodysplastic syndrome (MDS) in combination with cytarabine and in patients with brain tumors in combination with temozolomide. After Onrigin was rejected by the Food and Drug Administration for an AML indication in 2009 due to an unfavorable risk-benefit profile, the company went defunct.
Two anticancer agents, Onrigin (laromustine), formerly cloretazine (VNP40101M), and Triapine, a ribunucloetide reductase inhibitor similar tohydroxyurea, were in human clinical trials. Onrigin (laromustine), a novel alkylating agent, was evaluated in a Phase 2 trial in elderly de novo poor-riskacute myeloid leukemia (AML). In addition, several trials of Onrigin (laromustine) were conducted in elderly patients with AML and myelodysplastic syndrome (MDS) in combination with cytarabine and in patients with brain tumors in combination with temozolomide. After Onrigin was rejected by the Food and Drug Administration for an AML indication in 2009 due to an unfavorable risk-benefit profile, the company went defunct.
Onrigin



