Idarubicin hydrochloride
NSC-256439, IMI-30, DMDR, Idamycin, Zavedos
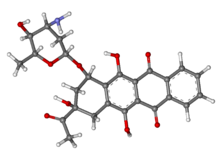
Idarubicin /ˌaɪdəˈruːbɨsɪn/ or 4-demethoxydaunorubicin is an anthracyclineantileukemic drug. It inserts itself into DNA and prevents DNA from unwinding by interfering with the enzyme topoisomerase II. It is an analog of daunorubicin, but the absence of a methoxy group increases its fat solubility and cellular uptake.[1] Similar to other anthracyclines, it also induces histone eviction fromchromatin.[2]
It belongs to the family of drugs called antitumor antibiotics.
It is currently combined with cytosine arabinoside as a first line treatment ofacute myeloid leukemia.
It is distributed under the trade names Zavedos (UK) and Idamycin (USA).
UV – spectrum
IR – spectrum
Brief background information
| SALT | ATC | FORMULA | MM | CAS |
|---|---|---|---|---|
| - | L01DB06 | C 26 H 27 NO 9 | 497.50 g / mol | 58957-92-9 |
Idarubicin is the 4-demethoxy derivative of daunorubicin. Idarubicin is an antineoplastic agent that has been used to treat various cancers, including those of the breast, lung, stomach, ovaries, and lymph system. Idarubicin is marketed as an intravenous injection of Idarubicin hydrochloride of the formula,
under the brand name IDAMYCIN®. Idarubicin hydrochloride is a red-orange crystalline powder, soluble in water, methanol, and other polar solvents like dimethylformamide. It is practically insoluble in acetone, chloroform, and methylene chloride. Idarubicin hydrochloride has a melting point of 175-180°C, and apH of 5.0-6.5 in a 0.5% w/v solution in water.
Application
- antitumor agent
- anthracycline antibiotic
Classes of substances
- Naftatsenovye antibiotics
Synthesis pathway
The reaction of daunomycinone (IX) with AlCl3 in dichloromethane gives 4-demethyldaunomycinone (X), which is ketalized with ethylene glycol as before yielding the dioxolane (XI). The selective sulfonation of (XI) with TsCl, DIEA and DMAP in pyridine affords the 4-tosyloxy derivative (XII), which is treated with 4-methoxybenzylamine (XIII) in pyridine providing the secondary benzylamine (XIV). Elimination of the benzyl protecting group of (XIV) with TFA gives 4-amino-4-demethoxydaunomycinone ethylene ketal (XV), which is deaminated by reaction with TFA, NaNO2 and H3PO2 to give 4-demethoxydaunomycinone (XVI). Finally, this compound is submitted to fermentation with Streptomyces peucetius corneus, S. Peucetius caesius, S. Caeruleus, S. Peucetius , S. Coeruleorubidus, and other chemical or radio-induced mutants thereof.

Mitscher, LA; Lednicer, D. (Pharmacia Corp.); Biosynthesis of simplified anthracyclines US 4471052.
condensation of chiral tetraline (I) with phthalic anhydride (II) by means of AlCl3 at 180 C gives the naphthacenedione (III), acetyl group which is ketalized with ethylene glycol and p-toluenesulfonic acid yielding the dioxolane (IV). The hydroxylation of (IV) with Br2 and AIBN in CCl4/CHCl3 affords the 4-demethoxy-7-epidaunomycinone (V), which is isomerized with TFA yielding 4-demethoxydaunomycinone (VI) . The condensation of (VI) with the acylated hexopyranosyl chloride (VII) by means of CF3SO3Ag of Br2Hg affords the trifluoroacetylated 4-demethoxydaunomycin (VIII), which is finally deprotected by treated with NaOH to eliminate the trifluoroacetyl groups
Trade Names
| COUNTRY | TRADE NAME | MANUFACTURER |
|---|---|---|
| Germany | Zavedos | Pharmacia |
| France | - “- | Pfizer |
| United Kingdom | - “- | Pharmacia |
| Italy | - “- | Pharmacia & Upjohn |
| Japan | Idamitsin | Pfizer |
| Ukraine | Zavedos | Actavis Italy SpA, Italy |
| Idalek | CJSC “Biolik”, Ukraine | |
| Zavedos | Pfizer Іtaliya Srl, Іtaliya | |
| Rubidium | NGO “Lance Farm”, Russia | |
| other generic drugs | ||
Formulations
- Capsules of 5 mg, 10 mg, 25 mg;
- vial of 5 mg, 10 mg (hydrochloride)
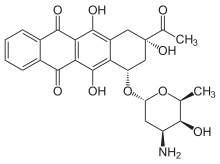
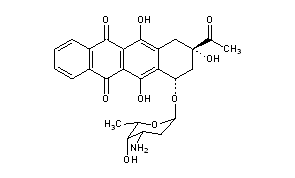
IDAMYCIN PFS Injection contains idarubicin hydrochloride and is a sterile, semi-synthetic, preservative-free solution (PFS) antineoplastic anthracycline for intravenous use. Chemically, idarubicin hydrochloride is 5, 12-Naphthacenedione, 9-acetyl-7-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxyhydrochloride, (7S-cis). The structural formula is as follows:
 |
C26H27NO9•Hcl M.W 533.96
IDAMYCIN PFS (idarubicin hydrochloride injection) is a sterile, red-orange, isotonic parenteral preservative-free solution, available in 5 mL (5 mg), 10 mL (10 mg) and 20 mL (20 mg) single-use-only vials.
Each mL contains Idarubicin HCL, USP 1 mg and the following inactive ingredients: Glycerin, USP 25 mg and Water for Injection, USP q.s. Hydrochloric Acid, NF is used to adjust the pH to a target of 3.5.
| Product Name | Idarubicin Hydrochloride |
| Chemical Name | (7S,9S)-9-Acetyl-7-[(3-amino-2,3,6-trideoxy-a-L- lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11- trihydroxy-5,12-naphthacenedione hydrochloride |
| Synonym | Idamycin; Zavedos |
| Formula Wt. | 533.96 |
| Melting Point | 183oC-185oC |
| Purity | ≥98% |
| Solubility | Soluble in water and methanol. |
| Store Temp | -20oC |
| References | Ganzina, F., Pacciarini, MA., Di Pietro, N. Invest New Drugs. 4:85-105 (1986). Tsuruo, T., Oh-Hara, T., Sudo, Y., Naito, M. Anticancer Res. 13:357-61 (1993). Belaud-Rotureau, MA., Durrieu, F., Labroille, G. et al Leukemia 14:1266-75 (2000). |
 | |
 | |
| SYSTEMATIC (IUPAC) NAME | |
|---|---|
| (1S,3S)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxo-α-L-lyxo-hexopyranoside | |
| CLINICAL DATA | |
| AHFS/DRUGS.COM | monograph |
| MEDLINEPLUS | a691004 |
| PREGNANCY CAT. | D (US) |
| LEGAL STATUS | ℞-only (US) |
| PHARMACOKINETIC DATA | |
| PROTEIN BINDING | 97% |
| HALF-LIFE | 22 hours |
| IDENTIFIERS | |
| CAS NUMBER | 58957-92-9 |
| ATC CODE | L01DB06 |
| PUBCHEM | CID 42890 |
| DRUGBANK | DB01177 |
| CHEMSPIDER | 39117 |
| UNII | ZRP63D75JW |
| KEGG | D08062 |
| CHEBI | CHEBI:42068 |
| CHEMBL | CHEMBL1117 |
| SYNONYMS | 9-acetyl-7-(4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl)oxy-6,9,11-trihydroxy-7,8,9,10-tetrahydrotetracene-5,12-dione |
| CHEMICAL DATA | |
| FORMULA | C26H27NO9 |
| MOL. MASS | 497.494 g/mol |
Links
- Synthesis a)
- US 4,471,052 (Adria; 9.11.1984; appl. 18.1.1982).
- Synthesis of b)
- DOS 2,525,633 (Soc. Farmaceutici; appl. 06.09.1975; GB -prior. 16.12.1974).
- US 4,046,878 (Soc. Farmaceutici; 09/06/1977; appl. 05/22/1975;GB -prior. 12.6.1974).
- UV and IR Spectra. H.-W. Dibbern, RM Muller, E. Wirbitzki, 2002 ECV
- NIST / EPA / NIH Mass Spectral Library 2008
- Handbook of Organic Compounds. NIR, IR, Raman, and UV-Vis Spectra Featuring Polymers and Surfactants, Jr., Jerry Workman.Academic Press, 2000.
- Handbook of ultraviolet and visible absorption spectra of organic compounds, K. Hirayama. Plenum Press Data Division, 1967.
References
- Package insert
- Pang B, Qiao X, Janssen L, Velds A, Groothuis T, Kerkhoven R, Nieuwland M, Ovaa H, Rottenberg S, van Tellingen O, Janssen J, Huijgens P, Zwart W, Neefjes J (2013). “Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin”. Nature Communications 4: 1908. doi:10.1038/ncomms2921.PMID 23715267.
External links
- Idarubicin bound to proteins in the PDB
 |
Title: Idarubicin
CAS Registry Number: 58957-92-9
CAS Name: (7S,9S)-9-Acetyl-7-[(3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxy-5,12-naphthacenedione
Additional Names: (1S,3S)-3-acetyl-1,2,3,4,6,11-hexahydro-3,5,12-trihydroxy-6,11-dioxo-1-naphthacenyl-3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranoside; 4-demethoxydaunomycin; 4-demethoxydaunorubicin; DMDR
Manufacturers' Codes: IMI-30; NSC-256439
Molecular Formula: C26H27NO9
Molecular Weight: 497.49
Percent Composition: C 62.77%, H 5.47%, N 2.82%, O 28.94%
Literature References: Orally active anthracycline; analog of daunorubicin, q.v. Prepn: B. Patelli et al. DE 2525633; eidem, US4046878 (1976, 1977 both to Soc. Farmac. Ital.); and antitumor activity: F. Arcamone et al., Cancer Treat. Rep. 60, 829 (1976). Total synthesis for larger scale preparation: M. J. Broadhurst et al., Chem. Commun. 1982, 158. Synthesis of optically pure isomers: Y. Kimura et al., Bull. Chem. Soc. Jpn. 59, 423 (1986). Metabolism and biodistribution in rats: G. Zini et al., Cancer Chemother. Pharmacol. 16, 107 (1986). HPLC determn in plasma: S. S. N. De Graaf et al., J. Chromatogr. 491, 501 (1989). Clinical pharmacokinetics: H. C. Gillies et al., Br. J. Clin. Pharmacol. 23, 303 (1987). Clinical evaluation of cardiac toxicity: F. Villani et al., Eur. J. Cancer Clin. Oncol. 25, 13 (1989). Reviews of pharmacology and antitumor efficacy: A. M. Casazza, Cancer Treat. Rep. 63, 835-844 (1979); F. Ganzina et al., Invest. New Drugs 4, 85-105 (1986). Symposium on clinical experience in acute leukemias: Semin. Oncol. 17, Suppl. 2, 1-36 (1989).
Derivative Type: Hydrochloride
CAS Registry Number: 57852-57-0
Trademarks: Idamycin (Pharmacia & Upjohn); Zavedos (Pharmacia & Upjohn)
Molecular Formula: C26H27NO9.HCl
Molecular Weight: 533.95
Percent Composition: C 58.48%, H 5.29%, N 2.62%, O 26.97%, Cl 6.64%
Properties: Orange crystalline powder, mp 183-185° (Arcamone); also reported as mp 172-174° (Broadhurst). [a]D20 +205° (c = 0.1 in methanol) (Arcamone); also reported as [a]D20 +188° (c = 0.10 in methanol) (Kimura).
Melting point: mp 183-185° (Arcamone); mp 172-174° (Broadhurst)
Optical Rotation: [a]D20 +205° (c = 0.1 in methanol) (Arcamone); [a]D20 +188° (c = 0.10 in methanol) (Kimura)
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic; Antibiotics and Analogs; Anthracyclines; Topoisomerase II Inhibitor.
|

















