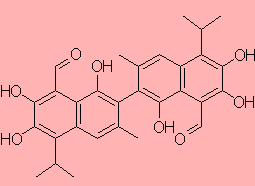

Gossypol
2,2-Bis(1,6,7-trihydroxy-3-methyl-5-isopropyl-8-formylnaphthalene)
This ridiculously named molecule is found in cotton seeds. It was used as a male contraceptive in China, but was never used in the West (and may have since been banned in China as well), since its effects were permanent in about 20% of patients! Its name originated from being present in the flowers of the Indian cotton plant Gossypium herbaceum L. Apart from its contraceptive effects, gossypol has properties that might make it useful in treating a number of ailments, including cancer, HIV, malaria and some bacterial/viral illnessness. Related to this molecule are the equally strangely named gossypetin and gossypin.
Among other things, it has been tested as a
male oral contraceptive in
China. In addition to its contraceptive properties, gossypol has also long been known to possess
antimalarial properties. Other researchers are investigating the anticancer properties of gossypol.
Biological properties
It has proapoptotic properties, probably due to the regulation of the Bax and Bcl2. It also reversibly inhibits calcineurin and binds to calmodulin. It inhibits replication of the HIV-1 virus.[1] It is an effective protein kinase C inhibitor.[2] It also causes low potassium levels, and thus causes temporary paralysis.
Biosynthesis
Gossypol is a terpenoid aldehyde, which is formed metabolically through acetate via the isoprenoid pathway.
[3] Sesquiterpene dimer undergoes a radical coupling reaction to form gossypol.
[4] Geranyl pyrophosphate (GPP) and IPP make sesquiterpene precursor, farnesyl diphosphate (FPP), for gossypol. The biosynthesis of gossypol is summarized in figure below. The biosynthesis begins with the 0 compound derived from GPP and IPP. Cadinyl cation (1) is oxidized to 2 by (+)-d-cadinene synthase. The (+)-d-cadinene (2) is involved in making the basic aromatic sesquiterpene unit, homigossypol, by oxidation, which generates the 3 (8-hydroxy-d-cadinene) with the help of (+)-d-cadinene 8-hyroxylase. At 5, the 3 goes through various oxidative processes to make 4 (deoxyhemigossypol), which is oxidized by one electron into hemigossypol (5,6,7) and then undergoes a phenolic oxidative coupling, ortho to the phenol groups, to form 8 (gossypol).
[5] The coupling is catalyzed by a hydrogen peroxide-dependent peroxidise enzyme, which results in the final product.
[5]
Contraceptive use
A 1929 investigation in
Jiangxi showed correlation between low fertility in males and use of crude
cottonseed oil for cooking. The compound causing the contraceptive effect was determined to be gossypol.
In the 1970s, the Chinese government began researching the use of gossypol as a
contraceptive. Their studies involved over 10,000 subjects, and continued for over a decade. They concluded gossypol provided reliable contraception, could be taken orally as a tablet, and did not upset men's balance of hormones.
However, gossypol also had serious flaws. The studies also discovered an abnormally high rate of
hypokalemia among subjects.
[6] Hypokalemia — low blood
potassium levels — causes symptoms of
fatigue, muscle weakness, and at its most extreme,
paralysis. In addition, about 7% of subjects
[citation needed] reported effects on their
digestive systems, and about 12%
[citation needed] had increased fatigue. Most subjects recovered after stopping treatment and taking potassium supplements. The same study showed taking potassium supplements during gossypol treatment did not prevent hypokalemia in
primates.
[7] The potassium deficiency may also be a result of the Chinese diet or genetic predisposition.
[8]
In the mid-1990s, the
Brazilian pharmaceutical company Hebron announced plans to market a low-dose gossypol pill called Nofertil, but the pill never came to market. Its release was indefinitely postponed due to unacceptably high rates of permanent infertility
[]. 5-25% of the men remained
azoospermic up to a year after stopping treatment.
[9] The longer the men had taken the drug and the higher their overall dosages, the more likely
[citation needed] they were to have lowered fertility or to become completely
infertile.
Researchers have suggested gossypol might make a good noninvasive alternative to surgical vasectomy.
[10]
In 1986, the Chinese stopped research because of these side effects.
In 1998, the
World Health Organization's Research Group on Methods for the Regulation of Male Fertility recommended the research should be abandoned. In addition to the other side effects, the WHO researchers were concerned about gossypol's toxicity: the toxic dose in primates is less than 10 times the contraceptive dose.
[11]This report effectively ended further studies of gossypol as a temporary contraceptive, but research into using it as an alternative to
vasectomy continues in
Austria,
Brazil,
Chile,
China, the
Dominican Republic, and
Nigeria.
First identified as an anti-fertility agent in China in the 1950s, gossypol is also a component of cottonseed oil, which is used for cooking. It's derived from the stems, roots, and seeds of plants from the Malvaceae family. The cotton plant (Gossypium species) is the most common source. Gossypol is the active ingredient found in seeds and other parts of the plant; however, content varies significantly from specjes to species.
Gossypol exerts antifertility action by inhibiting sperm production and motility. It possesses antitumorigenic activity and may also have anti-human immunodeficiency virus properties. It's available as liquid extracts and tinctures.
Reported uses
Gossypol is used in China as a male contraceptive. It's also used topically as a spermicide.
Administration
Dosage is 20 mg by mouth every day for 2 to 3 months until the sperm count is decreased to less than 4 million/ml. The dosage is reduced to a maintenance ranging from 50 mg weekly to 75 to 100 mg twice a month.
Hazards
Adverse effects associated with gossypol include paralysis, circulatory problems, diarrhea, malnutrition, hypokalemia, muscle fatigue, muscle weakness, and hair discoloration.
Pregnant and breast-feeding patients shouldn't use this herb. Patients with renal insufficiency should use with caution.
Safety Risk Gossypol has been associated with heart failure and nephrotoxicity. There's increased risk of nephrotoxicity when gossypol is given with nephrotoxic drugs. Administration with potassium-wasting diuretics could lead to hypokalemia.
Clinical considerations
The contraceptive effect of gossypol in men is higher than 99%. Fertility usually returns to normal within 3 months of discontinuation; however, inhibition of spermatogenesis may persist in up to 20% of men 2 years after discontinuation.
Monitor serum electrolyte levels, especially potassium, creatinine, and BUN levels.
Monitor patient for muscle weakness and fatigue.
If using formulation containing alcohol, avoid using in patients taking disulfiram, metronidazole, cephalosporins, or any CNS depressants.
If patient is pregnant or breast-feeding, advise her not to use gossypol.
Inform men of the potential for permanent sterility after using oral gossypol.
Food and animal agricultural industries must manage cotton-derivative product levels to avoid toxicity. For example, only
ruminant microflora can digest gossypol, but only to a certain level, and cottonseed oil must be refined.
A research team at
Texas A&M University has genetically engineered cotton plants that contain very little gossypol in the seed, but still contain the compound in the stems and leaves. This provides protection against pests and diseases, while allowing the seed to be used for oil and meal for human consumption. The plants are modified by
RNA interference, shutting down the genes for gossypol production in the seed, while leaving them unaffected in the rest of the plant. The resulting gossypol-free cottonseed is then suitable as a high-quality
protein source suitable for consumption not only by cattle, but also by humans. Protein makes up 23% of the cottonseed.
[12][13]
Gossypol is toxic to
erythrocytes in vitro by stimulating cell death contributing to the side effect of hemolytic anemia.
[14]
References[edit]
- Jump up^ Polsky, B; Segal, SJ; Baron, PA; Gold, JW; Ueno, H; Armstrong, D (1989). "Inactivation of human immunodeficiency virus in vitro by gossypol". Contraception 39 (6): 579–87.doi:10.1016/0010-7824(89)90034-6. PMID 2473865.
- Jump up^ Gossypol (Gossipol). Bioscreening.net (last updated 2008-07-09). Retrieved on 2012-06-09.
- Jump up^ Burgos, M.; Ito, S.; Segal, J. S.; Tran, T. P. (1997). "Effect of Gossypol on Ultrastructure of Spisula Sperm". Biol. Bull. 193: 228–229.
- Jump up^ Heinstein, P. F. ; Herman, L. D.; Tove, B. S.; Smith, H. F. (1970). "Biosynthesis of Gossypol". J. Biol. Chem. 245 (18): 4658–4665. PMID 4318479.
- ^ Jump up to:a b Dewick, P. M. Medicinal Natural Product: A Biosynthetic approach. 3rd ed., 2008 ISBN 0-470-74167-8
- Jump up^ Gossypol. Malecontraceptives.org (2011-07-27). Retrieved on 2013-03-30.
- Jump up^ Gossypol. Malecontraceptives.org (2011-07-27). Retrieved on 2013-03-30.
- Jump up^ Gossypol. Malecontraceptives.org (2011-07-27). Retrieved on 2013-03-30.
- Jump up^ Gossypol. Malecontraceptives.org (2011-07-27). Retrieved on 2013-03-30.
- Jump up^ Coutinho, F. M. (2002). "Gossypol: a contraceptive for men". Contraception 65 (4): 259–263. doi:10.1016/S0010-7824(02)00294-9. PMID 12020773.
- Jump up^ Gossypol. Malecontraceptives.org (2011-07-27). Retrieved on 2013-03-30.
- Jump up^ Cottonseed Protein: From Farmers to Your Family Table. Medgadget.com (2006-11-22). Retrieved on 2012-06-09.
- Jump up^ Walsh, Brian. Hungry? How About Some Protein-Rich Cotton..., Time Magazine, September 14, 2009, p. 54
- Jump up^ Zbidah, M; Lupescu, A; Shaik, N; Lang, F (2012). "Gossypol-induced suicidal erythrocyte death". Toxicology 302 (2–3): 101. doi:10.1016/j.tox.2012.09.010. PMID 23041711.

- Ibragimov, B. T.; Talipov, S. A.; Aripov, T. F.; Sadykov, A. S. (1990). "Inclusion complexes of the natural product gossypol. Crystal structure of the 2:1 complex of gossypol withm-Xylene". Journal of Inclusion Phenomena and Molecular Recognition in Chemistry 8 (3): 323. doi:10.1007/BF01041888.
Biosynthesis of gossypol from hemigossypol (Stipanovic et. al. 2005)
the compound is derived from hemigossypol through a free radical coupling reaction (Stipanovic et. al. 2005). This coupling reaction produces the two enantiomers (+)-gossypol and (-)-gossypol, depending on the orientation around the binaphthyl bond.
The toxicity of gossypol has made it an area of interest in plant research. When ingested orally, its toxicity can lead to anorexia, severe weight loss, and even death (Eagle 1949). The (-)-gossypol enantiomer will enter the cell and act as an inhibitor of dehydrogenase enzymes involved electron transport (Benz et. al. 1990), which ultimately leads to necrosis of the tissue.
There are also advantageous properties of gossypol to the cotton plant, especially in medicinal uses. (-)-gossypol is a potential aid in cancer research due to its inhibition of cell growth (Band et. al. 1989; Blackstaffe et. all 1997; Shelley et. al. 1999). This is accomplished by reduction of ATP by the uncoupling of oxidative phosphorylation in electron transport (Benz et. al. 1990). HIV research has shown that (-)-gossypol shows anti-HIV-1 activity (Lin et. al. 1993).This enantiomer can be used as an antiamoebic agent (Gonzales-Graza et. al. 1992). There have also been studies in China to suggest its use as a male contraceptive (Matlin et. al. 1985; Lindberg et. al. 1987; Wang et. al. 1987; Yao et. al. 1987).
Research shows that (+)-gossypol is useful to cotton as a natural protection against insects and pathogens. For example, Helicoverpa armigera larvae mature slowly and have a lower survival rate on cotton plants with (+)-gossypol (Yang et. al. 1999). It can also be consumed by nonruminant animals without expressing any signs of toxicity (Stipanovic et. al. 2005).
Cotton is well known as the leading fiber crop in the world (Lusas and Jividen 1987), but it's potential as a food crop has often been overlooked (Alford et. al. 1996). It has actually been classified as the second best potential source for plant proteins and the fifth best oil-producing plant (Texier 1993). Gossypol's toxicity restrains the potential of using cotton as a food crop, which is why emphasis has been placed on breeding for cotton with reduced amounts of gossypol in its seed (Vroh et. al. 1999). With low gossypol in the seed it can be safely used to produce cotton oil.
Zerp et al. Radiation Oncology 2009 4:47 doi:10.1186/1748-717X-4-47
Synonym: AT 101; 1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-2,2'-binaphthyl-8,8'-dicarbaldehyde
Application: An inhibitor of PKC, Bcl-2, 5-LO, and 12-LO
CAS Number: 303-45-7
Purity: ≥90%
Molecular Weight: 518.56
Molecular Formula: C30H30O8
Set Currency Add Selected
Description
Gossypol is a male antifertility agent with antispermatogenic activity and has been shown to contain anitumor, anitviral, and antioxidant properties. Gossypol is a reversible inhibitor of PP2B (protein phosphatase 2B), mitotic kinesin Eg5, and PKC (protein kinase C). Gossypol also potentially inhibits PAF-R, Bcl-2, 5-LO (5-lipoxygenase), 12-LO, and leukotriene-induced guinea pig parenchyma contractions. Gossypol is an inhibitor of Bcl-xl, PKD and p107.
Technical Information
Appearance: Off-white to yellow crystalline solid
Physical State: Solid
Derived from: Gossypium genus, Malvaceae
Solubility: Soluble in 100%ethanol (25 mg/ml), DMF (25 mg/ml), acetone, DMSO (25 mM), methanol (2 mg/ml), ether, chloroform, sodium carbonate, and dilute aqueous solutions of ammonia . Insoluble in water.
Storage: Store at -20° C
Melting Point: 191-193 ºC
Boiling Point: 707.89 °C at 760 mmHg
Density: 1.40 g/cm3
Refractive Index: n20D 1.74
IC50: mitotic kinesin Eg5: IC50 = 10.8 µM; RBL-1, 5-lipoxygenase: IC50 = 0.3 µM; 12-lipoxygenase: IC50 = 0.7 µM; PC-3 (Prostate carcinoma cells) : EC5050 = 17.8 µM (Homo sapiens ); PC-3 (Prostate carcinoma cells) : EC5050 = 3.3 µM (Homo sapiens ); Transitional endoplasmic reticulum ATPase : IC50 = 5.39 µM (Homo sapiens ); Induced myeloid leukemia cell differentiation protein Mcl-1 : IC50 = 1.36 µM (Homo sapiens ); Estrogen receptor beta : IC50 >50 µM (Homo sapiens ); Apoptosis regulator Bcl-2 : IC50 = 0.5 µM (Homo sapiens ); HCT-116 (Colon carcinoma cells) : IC50 = 3.3 µM (Homo sapiens ); Malate dehydrogenase mitochondrial Malate dehydrogenase mitochondrial : IC50 = 2.8 µM (Sus scrofa ); MDA-MB-231 (Breast adenocarcinoma cells) : IC50 = 1.9 µM (Homo sapiens ); Lactate dehydrogenase : IC50 = 2.64 µM (Plasmodium falciparum ); Malate dehydrogenase : IC50 = 2.03 µM (Plasmodium falciparum )
Ki Data: Induced myeloid leukemia cell differentiation protein Mcl-1 : Ki= 0.18 µM (Homo sapiens ); Apoptosis regulator Bcl-X : Ki= 0.48 µM (Homo sapiens ); Apoptosis regulator Bcl-2 : Ki= 0.32 µM (Homo sapiens ); Aldose reductase : Ki= 0.5 µM (Homo sapiens ); Apoptosis regulator Bcl-2 : Ki= 0.17 µM (Homo sapiens ); Apoptosis regulator Bcl-W : Ki= 17.7 µM (Homo sapiens )


3,4-Dimethoxy-2-isopropylbenzaldehyde (I) could be condensed with diethyl succinate (II) to produce 2-(2-isopropyl-3,4-dimethoxybenzylidene)succinic acid monoethyl ester (III) in the presence of NaH in refluxing benzene, and the yielding product is cyclized by treatment with acetic anhydride and sodium acetate in refluxing acetic acid and hydrolyzed with NaOH in refluxing methanol affording acid (IV). Next, (IV) is reduced with LiAlH4 in ether to give 3-hydroxymethyl-5-isopropyl-6,7-dimethoxy-1-naphthol (V), and the compound produced is hydrogenated with H2 over Pd/C in methanol with some HCl affording 3-methyl-5-isopropyl-6,7-dimethoxy-1-naphthol (VI). Thermal dimerization of (VI) in the condition of heating at 215 C yields 2,2-bis(3-methyl-5-isopropyl-6,7-dimethoxy-1-naphthol) (VII).















