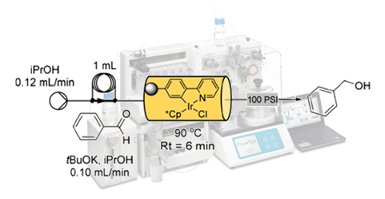
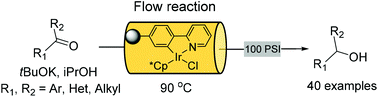
Flow synthesis offers many advantages when applied to the processing of difficult or dangerous chemical transformations. Furthermore, continuous production allows for rapid scale up of reactions without significant redevelopment of the routes. Importantly, it can also provide a versatile platform from which to build integrated multi-step transformations, delivering more advanced chemical architectures. The construction of multi-purpose micro and meso flow systems, that utilize in-line purification and diagnostic capabilities, creates a scenario of seamless connectivity between sequential steps of a longer chemical sequence. In this mini perspective, we will discuss our experience of target orientated multi-step synthesis as presented at the recent inaugural meeting of LEGOMEDIC at Namar University, Belgium.
The true potential of flow chemistry as an enabling technology can really only be fully appreciated when seen in the context of a target driven multi-step synthesis, aimed at the delivery of advanced chemical structures such as active pharmaceutical ingredients (APIs) .
As most pharmaceutical syntheses typically require between 8 and 10 chemical transformations (this is often somewhat reduced to 5/6 steps when analogue/library syntheses are being conducted), excluding protecting group manipulations, to realize the target molecule, this is a good foundation from which to explore the advantages of flow chemistry. We have generated a flow protocol for the synthesis of imatinib, the API of the Novartis block buster anticancer therapeutic Gleevec (imatinib mesylate), including a series of analogues (
Scheme 11)
Furthermore, we aimed to create a route which would allow each of the three main fragments to be exchanged to address maximum variation in subsequent analogue synthesis. This requires additional planning to build flexibility into the sequence where this desired diversity can be easily introduced. Again, prior consideration of the generated intermediates, and any potential by-products that may arise, is critical and should be addressed prior to embarking on the synthesis.
Consequently, the extensive profiling of the reaction in terms of its purity profile is more closely analogous to process chemistry than traditional Medicinal Chemistry, even at the development stage. So, although more time consuming in the planning stage, having a greater understanding of the chemistry, does then enable a smoother up scaling and more rapid optimization of the route.

Scheme 11Flow route used to prepare imatinib related analogues.
read all this at
Flow chemistry approaches directed at improving chemical synthesis
1Department of Chemistry, Durham University, South Road, Durham, DH1 3LE, UK
Corresponding author: Ian R. Baxendale, Department of Chemistry, Durham University, South Road, Durham, DH1 3LE, UK
Citation Information: Green Processing and Synthesis. Volume 2, Issue 3, Pages 211–230, ISSN (Online) 2191-9550, ISSN (Print) 2191-9542, DOI:
10.1515/gps-2013-0029, May 2013