
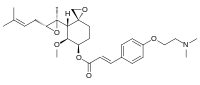
Beloranib
CAS 251111-30-5 (beloranib),529511-79-3 (beloranib hemioxalate)
(E)-(3R,4S,5S,6R)-5-methoxy-4-((2R,3R)-2-methyl-3-(3-methylbut-2-en-1-yl)oxiran-2-yl)-1-oxaspiro[2.5]octan-6-yl 3-(4-(2-(dimethylamino)ethoxy)phenyl)acrylate
6-O-(4-dimethylaminoethoxy)cinnamoyl fumagillol
Mechanism of Action:methionine aminopeptidase 2 (MetAP2) inhibitor
Indication:Obesity US Patent : US6063812 Patent Exp Date: May 13, 2019
Originator: Chong Kun Dang (CKD) Pharma (종근당) Chong Kun Dang Pharm Corp
Developer: Zafgen Inc. (자프젠)Zafgen Corporation
Zafgen’s Prader-Willi syndrome therapy receives orphan drug designation in Europe The European Commission (EC) has granted orphan drug designation to US-based Zafgen for its beloranib for treating Prader-Willi syndrome. Beloranib is a potent inhibitor of Methionine aminopeptidase-2 that reduces hunger while stimulating the use of stored fat as an energy source (MetAP2). MetAP2 is an enzyme that modulates the activity of key cellular processes that control metabolism. http://www.pharmaceutical-technology.com/news/newszafgens-prader-willi-syndrome-therapy-receives-orphan-drug-designation-in-europe-4316842?WT.mc_id=DN_News
INTRODUCTION Beloranib is an experimental drug candidate for the treatment of obesity. It was discovered by CKD Pharmaceuticals and is currently being developed by Zafgen. Beloranib, an analog of the natural chemical compound fumagillin, is an inhibitor of the enzyme METAP2. It was originally designed as angiogenesis inhibitor for the treatment of cancer. However, once the potential anti-obesity effects of METAP2 inhibition became apparent, the clinical development began to focus on these effects and beloranib has shown positive results in preliminary clinical trials for this indication. At such low doses, says Thomas E. Hughes, president and chief executive officer of Zafgen, toxicity concerns tend to evaporate, in part because so little opportunity exists to inhibit off-target proteins.
Zafgen, a small pharmaceutical company in Cambridge, Mass., sees high selectivity and low toxicity with its covalent molecule for treating obesity, beloranib hemioxalate, also known as ZGN-433. “You’re passing a wave of the molecule through the body,” he says. “It hits the different tissues, silences the target enzyme where it finds it, and then it goes away.” Zafgen’s drug candidate inhibits an enzyme called methionine aminopeptidase 2 (MetAP2), which had been of interest in oncology circles until it turned out to be a poor target for treating cancer in mice. However, animals treated with a MetAP2 inhibitor lost weight. Zafgen pursued the enzyme as a target for obesity. Its drug candidate contains a spiroepoxide that bonds with a histidine in the protein’s active site.
ZGN-433 has undergone a Phase I clinical trial, in which obese volunteers lost up to 2 lb per week. It will enter Phase II trials within a year, Hughes says, funded by $33 million the company raised from investors. With dosing of up to 2 mg twice per week, ZGN-433 reaches a maximum concentration in the body of just a few nanomolar for several hours before the body quickly eliminates it, Hughes says. During that time, the drug is much more likely to interact with MetAP2 than with anything else. “You’re flying under the radar of a lot of concerns,” he says. “Drug-drug interactions are not an issue. There’s just not enough inhibitor to go around.
The same is true for off-target inhibition: The chance of off-target toxicity is largely gone.” Proponents of covalent inhibitors are quick to point out that dozens of such drugs are already on the market. They include aspirin, the world’s most widely used medicine; penicillin and related antibiotics; and recently developed blockbusters such as Plavix, Prevacid, and Nexium. The drugs treat a broad range of conditions, and many have minimal side effects, even when taken for years. By one count, of the marketed drugs that inhibit enzymes, more than one-third work by covalent modification (Biochemistry,DOI: 10.1021/bi050247e).

6-O-(4-dimethylaminoethoxy) cinnamoyl fumagillol hemioxalate
| BELORANIB, ZGN-433, CKD-732 | |
|---|---|
 | |
| IDENTIFIERS | |
| CAS number | 251111-30-5 |
| PubChem | 6918502 |
| ChemSpider | 26286923 |
| UNII | FI471K8BU6 |
| Jmol-3D images | Image 1 |
| PROPERTIES | |
| Molecular formula | C29H41NO6 |
| Molar mass | 499.64 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
Beloranib (previously known as CKD-732; ZGN-433), a methionine aminopeptidase 2 (MetAP2) inhibitor originally designed as an anticancer agent, is being developed by Zafgen as a first-in-class obesity therapy. Beloranib, a twice-daily injection, is discovered by korean company Chong Kun Dang (CKD) Pharmaceuticals and was licensed to Cambridge, MA-based startup Zafgen, Inc. Zafgen holds exclusive worldwide rights (exclusive of Korea) for development and commercialization of beloranib. Beloranib, an analog of the antimicrobial agent fumagillin, is an inhibitor of the enzyme METAP2 involved in fatty acid production. It was originally designed as angiogenesis inhibitor for the treatment of cancer. However, once the potential anti-obesity effects of METAP2 inhibition became apparent, the clinical development began to focus on these effects.
Zafgen has chosen to develop beloranib not for the folks that need to shed a few pounds, but for severely obese people, and smaller groups of patients with rare and dangerous conditions. In January 2013, beloranib was granted orphan drug designation by the U.S. Food and Drug Administration to treat a rare genetic condition known as Prader-Willi Syndrome (PWS) that causes obesity through compulsive eating. Zafgen plans to seek the same designation for beloranib in craniopharyngioma (a rare benign brain tumor) related obesity as well. By going after these orphan indications, Zafgen can get onto the market quicker and cheaper than if it went straight for the larger obesity market. Zafgen recently completed two Phase 2a clinical trials evaluating beloranib’s ability to reduce body weight and to improve hyperphagia, one in PWS patients and one in severely obese patients. In its Phase 2a clinical trials, Zafgen observed reductions in body weight, body mass and body fat content in both patient populations and reductions in hyperphagia-related behaviors in PWS patients.
On June 19, 2014, Zafgen Inc. raised $96 million in its initial public offering (IPO) on the Nasdaq under the symbol “ZFGN” amid strong demand from investors. With its IPO cash, Zafgen plans to initiate its Phase 3 clinical program, consisting of two Phase 3 clinical trials, of beloranib in PWS patients, with the first Phase 3 trial to start in the second half of 2014, after finalizing the program design based on ongoing conversations with the FDA and certain European regulatory authorities. Zafgen is also planning a phase 2a trial in craniopharyngioma, and a Phase 2b trila in patients with severe obesity, all this year. The composition of matter patent (US6063812) on beloranib will each expire in May 2019. Zafgen owns two issued U.S. patents relating to beloranib polymorph compositions of matter that will expire in 2031 and two issued U.S. patents to methods of treating obesity that will expire in 2029.  Beloranib is an experimental drug candidate for the treatment of obesity. It was discovered by CKD Pharmaceuticals and is currently being developed by Zafgen.[1] Beloranib, an analog of the natural chemical compound fumagillin, is aninhibitor of the enzyme METAP2.[2] It was originally designed asangiogenesis inhibitor for the treatment of cancer.[3] However, once the potential anti-obesity effects of METAP2 inhibition became apparent, the clinical development began to focus on these effects and beloranib has shown positive results in preliminary clinical trials for this indication.[4][5]
Beloranib is an experimental drug candidate for the treatment of obesity. It was discovered by CKD Pharmaceuticals and is currently being developed by Zafgen.[1] Beloranib, an analog of the natural chemical compound fumagillin, is aninhibitor of the enzyme METAP2.[2] It was originally designed asangiogenesis inhibitor for the treatment of cancer.[3] However, once the potential anti-obesity effects of METAP2 inhibition became apparent, the clinical development began to focus on these effects and beloranib has shown positive results in preliminary clinical trials for this indication.[4][5]
 Beloranib is an experimental drug candidate for the treatment of obesity. It was discovered by CKD Pharmaceuticals and is currently being developed by Zafgen.[1] Beloranib, an analog of the natural chemical compound fumagillin, is aninhibitor of the enzyme METAP2.[2] It was originally designed asangiogenesis inhibitor for the treatment of cancer.[3] However, once the potential anti-obesity effects of METAP2 inhibition became apparent, the clinical development began to focus on these effects and beloranib has shown positive results in preliminary clinical trials for this indication.[4][5]
Beloranib is an experimental drug candidate for the treatment of obesity. It was discovered by CKD Pharmaceuticals and is currently being developed by Zafgen.[1] Beloranib, an analog of the natural chemical compound fumagillin, is aninhibitor of the enzyme METAP2.[2] It was originally designed asangiogenesis inhibitor for the treatment of cancer.[3] However, once the potential anti-obesity effects of METAP2 inhibition became apparent, the clinical development began to focus on these effects and beloranib has shown positive results in preliminary clinical trials for this indication.[4][5]
………………………………..
compound O-(4- dimethylaminoethoxycinnamoyl)fumagillol can be used in the form of a salt, e.g., acetate, lactate, benzoate, salicylate, mandelate, oxalate, methanesulfonate, or p- toluenesulfonate. Korean Patent No. 0357542 and its corresponding patents (U.S. Patent No. 6,063,812, Japanese Patent No. 3370985, and European Patent No. 1077964), filed by the present applicant, disclose fumagiUol derivatives, including the compounds used in the present invention. The composition of the present invention can be prepared in combination with pharmaceutically acceptable carriers commonly used in pharmaceutical formulations.
………………………..
MetAP2 encodes a protein that functions at least in part by enzymatically removing the amino terminal methionine residue from certain newly translated proteins, such as, glyceraldehyde-3- phosphate dehydrogenase (Warder et al. (2008) J Proteome Res 7:4807). Increased expression of the MetAP2 gene has been historically associated with various forms of cancer. Molecules inhibiting the enzymatic activity of MetAP2 have been identified and have been explored for their utility in the treatment of various tumor types (Wang et al. (2003) Cancer Res 63:7861) and infectious diseases, such as, microsporidiosis, leishmaniasis, and malaria (Zhang et al. (2002) J. Biomed Sci. 9:34). Notably, inhibition of MetAP2 activity in obese and obese-diabetic animals leads to a reduction in body weight in part by increasing the oxidation of fat and in part by reducing the consumption of food (Rupnick et al. (2002) Proc Natl Acad Sci USA 99: 10730). [0003] 6-O-(4-Dimethylaminoethoxy)cinnamoyl fumagillol is a METAP2 inhibitor and is useful in the treatment of, e.g., obesity. 6-O-(4-Dimethylaminoethoxy)cinnamoyl fumagillol is characterized by formula I:
Example 1 [0060] Crystalline, Form A material of 6-O-(4-dimethylaminoethoxy)cinnamoyl fumagillol was prepared as follows: [0061] Approximately 423 mg of amorphous gum/oil-like 6-O-(4- dimethylaminoethoxy)cinnamoyl fumagillol free base compound was dissolved in ca. 6 mL of diisopropylether (IPE). The solution was allowed to stir for ca. 24 hours at ambient temperature (18-22°C) during which time solid precipitated. The resulting solid was isolated by filtration and dried under vacuum at ambient for ca. 4 hours (yield 35.8 %).
…………………..
………………….
Example 14 : 0-(4-dimethylaminocinnamoyl)fumagillol 1) To a solution of 4-dimethylaminocinnamic acid (950 mg) in toluene (20 ml), dipyridyl disulfide (1.64 g) and triphenyl phosphine (1.97 g) were added, and the mixture was stirred for 12 hours. 2) The resultant solution of 1) was added to fumagillol (500 mg) at room temperature. Sodium hydride (142 mg) was added thereto, and the reaction mixture was stirred for 30 minutes. After adding saturated ammonium chloride solution (20 ml), the reaction mixture was extracted with ethyl acetate (100 ml). The organic layer was washed with brine and dried over anhydrous magnesium sulfate. After filtering, the solvent was distilled off under reduced pressure, and the residue was purified by column chromatography (eluent: ethyl acetate/ n-hexane = 1/2) to obtain yellow solid (470 mg). ‘H-NMR (CDCI3) δ : 7.60 (d, IH, J=15.8Hz), 7.41 (d, 2H, J=8.9Hz), 6.67 (d, 2H, J=8.9Hz), 6.27 (d, IH, J=15.8Hz), 5.71 (m, IH), 5.22 (bit, IH), 3.70 (dd, IH, J=2.8, 11.0Hz), 3.45 (s, 3H), 3.02 (s, 6H), 3.01 (d, IH, J=4.3Hz), 2.63 (t, IH, J=6.3Hz), 2.56 (d, IH, J=4.3Hz), 2.41 – 1.81 (m, 6H), 1.75 (s, 3H), 1.67 (s, 3H), 1.22 (s, 3H), 1.15 – 1.06 (m, IH)
………..
Organic Letters, 16(3), 792-795; 2014

An efficient, two-step construction of highly complex alkaloid-like compounds from the natural product fumagillol is described. This approach, which mimics a biosynthetic cyclase/oxidase sequence, allows for rapid and efficient structure elaboration of the basic fumagillol scaffold with a variety of readily available coupling partners. Mechanistic experiments leading to the discovery of an oxygen-directed oxidative Mannich reaction are also described.
References
- “News Release: Zafgen Secures $33 Million Series C Financing”. Zafgen, Inc. July 7, 2011.
- Chun, E; Han, CK; Yoon, JH; Sim, TB; Kim, YK; Lee, KY (2005). “Novel inhibitors targeted to methionine aminopeptidase 2 (MetAP2) strongly inhibit the growth of cancers in xenografted nude model”. International Journal of Cancer. Journal International Du Cancer 114 (1): 124–30.doi:10.1002/ijc.20687. PMID 15523682.
- Kim, EJ; Shin, WH (2005). “General pharmacology of CKD-732, a new anticancer agent: effects on central nervous, cardiovascular, and respiratory system”. Biological & Pharmaceutical Bulletin 28 (2): 217–23.doi:10.1248/bpb.28.217. PMID 15684472.
- “Zafgen Announces Positive Topline Phase 1b Data for ZGN-433 in Obesity”. MedNews. Drugs.com. 5 January 2011.
- “Fat-busting pill helps obese to shed two pounds a week – without changing their diets”. UK Daily Mail. 11 January 2011.
MORE REF Grenning, Alexander J. et al.Remodeling of Fumagillol: Discovery of an Oxygen-Directed Oxidative Mannich Reaction.Organic Letters, 16(3), 792-795; 2014
Hughes, T. E.; Kim, D. D.; Marjason, J.; Proietto, J.; Whitehead, J. P.; Vath, J. E. Ascending dose-controlled trial of beloranib, a novel obesity treatment for safety, tolerability, and weight loss in obese women. Obesity (2013), 21(9), 1782-1788.
Chung Il Hong, Jung Woo Kim, Sang Joon Lee, Soon Kil Ahn, Nam Song Choi, Ryung Kee Hong, Hyoung Sik Chun, Seung Kee Moon, Cheol Kyu Han. Angiogenesis inhibitors, antiarthritic agents and anticarcinogenic agents plus synthesis. US patent Number US6063812 A, Also published as CA2331873A1, CA2331873C, CN1301260A, CN100352810C, DE69903279D1, DE69903279T2, EP1077964A1,EP1077964B1,WO1999059986A1, Filing date: May 13, 1999.Original Assignee:Chong Kun Dang Corporation Crawford, Thomas; Reece, Hayley A.Preparation of crystalline forms of 6-O-(4-dimethylaminoethoxy)cinnamoylfumagillol.PCT Int. Appl. (2012), WO2012064838 A1, 20120518
Egorov, Maxim et al. Preparation of fumagillol derivatives useful for the treatment or prevention of bone tumors. PCT Int. Appl., WO2012130906, 04 Oct 2012
Stevenson, Cheri A.; Akullian, Laura C.; Petter, Russell C.; Kane, John J.; Hammond, Charles E.; Yin, Mao; Yurkovetskiy, Aleksandr.Preparation of biocompatible biodegradable fumagillin analog conjugates for the treatment of cancer. PCT Int. Appl. (2009), WO2009073445 A2, 20090611
Lee, Hong Woo et al.Design, synthesis, and antiangiogenic effects of a series of potent novel fumagillin analogues.Chemical & Pharmaceutical Bulletin, 55(7), 1024-1029; 2007
Lee, Hong Woo et al.Selective N-demethylation of tertiary aminofumagillols with selenium dioxide via a non-classical Polonovski type reaction.Heterocycles, 68(5), 915-932; 2006
References OTHERS
|
1: Yin SQ, Wang JJ, Zhang CM, Liu ZP. The development of MetAP-2 inhibitors in cancer treatment. Curr Med Chem. 2012;19(7):1021-35. Review. PubMed PMID: 22229417.
2: Shin SJ, Ahn JB, Park KS, Lee YJ, Hong YS, Kim TW, Kim HR, Rha SY, Roh JK, Kim DH, Kim C, Chung HC. A Phase Ib pharmacokinetic study of the anti-angiogenic agent CKD-732 used in combination with capecitabine and oxaliplatin (XELOX) in metastatic colorectal cancer patients who progressed on irinotecan-based chemotherapy. Invest New Drugs. 2012 Apr;30(2):672-80. doi: 10.1007/s10637-010-9625-x. Epub 2010 Dec 29. PubMed PMID: 21188464.
3: Shin SJ, Jeung HC, Ahn JB, Rha SY, Roh JK, Park KS, Kim DH, Kim C, Chung HC. A phase I pharmacokinetic and pharmacodynamic study of CKD-732, an antiangiogenic agent, in patients with refractory solid cancer. Invest New Drugs. 2010 Oct;28(5):650-8. doi: 10.1007/s10637-009-9287-8. Epub 2009 Jul 8. PubMed PMID: 19585083.
4: Rhee Y, Park SY, Kim YM, Lee S, Lim SK. Angiogenesis inhibitor attenuates parathyroid hormone-induced anabolic effect. Biomed Pharmacother. 2009 Jan;63(1):63-8. doi: 10.1016/j.biopha.2007.10.013. Epub 2007 Nov 20. PubMed PMID: 18457934.
5: Kim YM, An JJ, Jin YJ, Rhee Y, Cha BS, Lee HC, Lim SK. Assessment of the anti-obesity effects of the TNP-470 analog, CKD-732. J Mol Endocrinol. 2007 Apr;38(4):455-65. PubMed PMID: 17446235.
6: Kim EJ, Shin WH. General pharmacology of CKD-732, a new anticancer agent: effects on central nervous, cardiovascular, and respiratory system. Biol Pharm Bull. 2005 Feb;28(2):217-23. PubMed PMID: 15684472.
7: Chun E, Han CK, Yoon JH, Sim TB, Kim YK, Lee KY. Novel inhibitors targeted to methionine aminopeptidase 2 (MetAP2) strongly inhibit the growth of cancers in xenografted nude model. Int J Cancer. 2005 Mar 10;114(1):124-30. PubMed PMID: 15523682.
8: Lee HS, Choi WK, Son HJ, Lee SS, Kim JK, Ahn SK, Hong CI, Min HK, Kim M, Myung SW. Absorption, distribution, metabolism, and excretion of CKD-732, a novel antiangiogenic fumagillin derivative, in rats, mice, and dogs. Arch Pharm Res. 2004 Feb;27(2):265-72. PubMed PMID: 15029870.
9: Kim JH, Lee SK, Ki MH, Choi WK, Ahn SK, Shin HJ, Hong CI. Development of parenteral formulation for a novel angiogenesis inhibitor, CKD-732 through complexation with hydroxypropyl-beta-cyclodextrin. Int J Pharm. 2004 Mar 19;272(1-2):79-89. PubMed PMID: 15019071.
10: Myung SW, Kim HY, Min HK, Kim DH, Kim M, Cho HW, Lee HS, Kim JK, Hong CI. The identification of in vitro metabolites of CKD-732 by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2002;16(21):2048-53. PubMed PMID: 12391579.
| WO2007072083A1 | Dec 22, 2006 | Jun 28, 2007 | Prosidion Ltd | Treatment of type 2 diabetes with a combination of dpiv inhibitor and metformin or thiazolidinedione |
| WO2011085201A1* | Jan 7, 2011 | Jul 14, 2011 | Zafgen Corporation | Fumagillol type compounds and methods of making and using same |
| WO2011088055A2* | Jan 11, 2011 | Jul 21, 2011 | Zafgen Corporation | Methods and compositions for treating cardiovascular disorders |
| WO2012064838A1 | Nov 9, 2011 | May 18, 2012 | Zafgen Corporation | Crystalline solids of a metap-2 inhibitor and methods of making and using same |
| WO2013169727A1* | May 7, 2013 | Nov 14, 2013 | Zafgen, Inc. | Polymorphic salt of the oxalate salt of 6 – o – ( 4 – dimethylaminoethoxy) cinnarnoyl fumagillol and methods of making and using same |
| WO2013169857A1* | May 8, 2013 | Nov 14, 2013 | Zafgen, Inc. | Treating hypothalamic obesity with metap2 inhibitors |
| EP2317845A1 * | Jul 17, 2009 | May 11, 2011 | Zafgen, Inc. | Methods of treating an overweight or obese subject |
| US8349891 | Aug 7, 2012 | Jan 8, 2013 | Zafgen, Inc. | Crystalline solids of a MetAP-2 inhibitor and methods of making and using same |
| US8367721 | Aug 7, 2012 | Feb 5, 2013 | Zafgen, Inc. | Methods of treating an overweight or obese subject |
| US8642650 | Dec 4, 2009 | Feb 4, 2014 | Zafgen, Inc. | Methods of treating an overweight or obese subject |
| US8735447 | Nov 16, 2012 | May 27, 2014 | Zafgen, Inc. | Crystalline solids of a MetAP-2 inhibitor and methods of making and using same |
| US20130018095 * | Jan 7, 2011 | Jan 17, 2013 | Vath James E | Fumigillol Type Compounds and Methods of Making and Using Same |
| WO2003027104A1* | Jun 11, 2002 | Apr 3, 2003 | Byung-Ha Chang | Fumagillol derivatives and preparing method thereof |
| EP0682020A1 * | Aug 31, 1989 | Nov 15, 1995 | Takeda Chemical Industries, Ltd. | Fumagillol derivatives useful as angiogenesis inhibitors |
| US6040337 * | May 13, 1999 | Mar 21, 2000 | Chong Kun Dang Corporation | 5-demethoxyfumagillol derivatives and processes for preparing the same |
| US6063812 * | May 13, 1999 | May 16, 2000 | Chong Kun Dang Corporation | Angiogenesis inhibitors, antiarthritic agents and anticarcinogenic agents plus synthesis |
| WO1999059986A1* | May 11, 1999 | Nov 25, 1999 | Soon Kil Ahn | Fumagillol derivatives and processes for preparing the same |
| WO2005082349A1 | Feb 25, 2005 | Sep 9, 2005 | Chong Kun Dang Pharm Corp | Composition for the treatment of obesity comprising fumagillol derivative |
| WO2010065883A2 | Dec 4, 2009 | Jun 10, 2010 | Zafgen Corporation | Method of treating an overweight or obese subject |
| KIM ET AL. JOURNAL OF MOLECULAR ENDOCRINOLOGY vol. 38, 2007, pages 455 – 465 | ||
| 2 | RUPNICK ET AL. PROC NATL ACAD SCI USA vol. 99, 2002, page 10730 | |
| 3 | WANG ET AL. CANCER RES vol. 63, 2003, page 7861 | |
| 4 | WARDER ET AL. J PROTEOME RES vol. 7, 2008, page 4807 | |
| 5 | * | YOO MEE KIM ET AL: “Assessment of the anti-obesity effects of the TNP-470 analog, CKD-732“, JOURNAL OF MOLECULAR ENDOCRINOLOGY, SOCIETY FOR ENDOCRINOLOGY, GB, vol. 38, no. 4, 1 April 2007 (2007-04-01), pages 455-465, XP002632891, ISSN: 0952-5041, DOI: 10.1677/JME.1.02165 |
| 6 | ZHANG ET AL. J. BIOMED SCI. vol. 9, 2002, page 34 |

