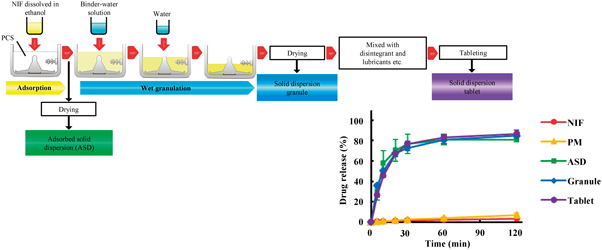
The aim of this study was to prepare and evaluate solid dispersion tablets containing a poorly water-soluble drug using porous calcium silicate (PCS) by a wet granulation method. Nifedipine (NIF) was used as the model poorly water-soluble drug. Solid dispersion tablets were prepared with the wet granulation method using ethanol and water by a high-speed mixer granulator. The binder and disintegrant were selected from 7 and 4 candidates, respectively. The dissolution test was conducted using the JP 16 paddle method. The oral absorption of NIF was studied in fasted rats. Xylitol and crospovidone were selected as the binder and disintegrant, respectively. The dissolution rates of NIF from solid dispersion formulations were markedly enhanced compared with NIF powder and physical mixtures. Powder X-ray diffraction (PXRD) confirmed the reduced crystallinity of NIF in the solid dispersion formulations. Fourier transform infrared (FT-IR) showed the physical interaction between NIF and PCS in the solid dispersion formulations. NIF is present in an amorphous state in granules prepared by the wet granulation method using water. The area under the plasma concentration–time curve (AUC) and peak concentration (Cmax) values of NIF after dosing rats with the solid dispersion granules were significantly greater than those after dosing with NIF powder. The solid dispersion formulations of NIF prepared with PCS using the wet granulation method exhibited accelerated dissolution rates and superior oral bioavailability. This method is very simple, and may be applicable to the development of other poorly water-soluble drugs.
The ‘Biopharmaceutics Classification System’ (BCS) is a very important key word in the research and development of oral formulations. The BCS classifies drugs into four classes depending on the solubility and membrane permeability of the drug. Most oral formulations show drug efficacy by first dissolving in the digestive tract then being absorbed through the membrane of the small intestine, thus entering the circulation. Oral formulations have been developed using various strategies depending on the drug’s BCS class, solubility, and membrane permeability. It was recently estimated that between 40 and 70% of all new chemical entities identified in drug discovery programs are insufficiently soluble in aqueous media.......... read all
Conclusion
Solid dispersion formulations of NIF with PCS using the wet granulation method were prepared and evaluated. These formulations exhibited much higher dissolution rates than NIF powder, comparable to ASD. Furthermore, these formulations provided superior bioavailability in rats compared with NIF powder. NIF was present in the amorphous state in the granules after preparation by a wet granulation method using water. The wet granulation method proposed here is very simple, and may be applicable to other poorly water-soluble drugs.
Preparation and Evaluation of Solid Dispersion Tablets by a Simple and Manufacturable Wet Granulation Method Using Porous Calcium Silicate
The ‘Biopharmaceutics Classification System’ (BCS) is a very important key word in the research and development of oral formulations. The BCS classifies drugs into four classes depending on the solubility and membrane permeability of the drug. Most oral formulations show drug efficacy by first dissolving in the digestive tract then being absorbed through the membrane of the small intestine, thus entering the circulation. Oral formulations have been developed using various strategies depending on the drug’s BCS class, solubility, and membrane permeability. It was recently estimated that between 40 and 70% of all new chemical entities identified in drug discovery programs are insufficiently soluble in aqueous media.......... read all
Conclusion
Solid dispersion formulations of NIF with PCS using the wet granulation method were prepared and evaluated. These formulations exhibited much higher dissolution rates than NIF powder, comparable to ASD. Furthermore, these formulations provided superior bioavailability in rats compared with NIF powder. NIF was present in the amorphous state in the granules after preparation by a wet granulation method using water. The wet granulation method proposed here is very simple, and may be applicable to other poorly water-soluble drugs.