Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, helping millions with websites, 9 million hits on google, 2.5 lakh connections worldwide, P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
Showing posts with label Riociguat. Show all posts
Showing posts with label Riociguat. Show all posts
Thursday, 26 September 2013
A Case of Idiopathic Pulmonary Hypertension
FDA grants two orphan drug designations for Bayer's Riociguat
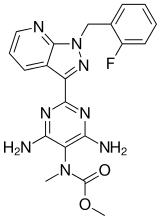
Riociguat
Bayer HealthCare Pharmaceuticals, a subsidiary of Bayer, has obtained two separate orphan drug designations from the US Food and Drug Administration's (FDA) Office of Orphan Products Development for its investigational, oral medication riociguat, proposed trade name Adempas.
read all at
http://regulatoryaffairs.pharmaceutical-business-review.com/news/fda-grants-two-orphan-drug-designations-for-bayers-riociguat-260913
Riociguat (BAY 63-2521) is a novel drug that is in clinical development by Bayer. It is a stimulator of soluble guanylate cyclase(sGC). At the moment Phase III clinical trials investigate the use of riociguat as a new approach to treat two forms of pulmonary hypertension (PH): chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension (PAH). Riociguat constitutes the first drug of a novel class of sGC stimulators.
The first nitric oxide (NO) independent, haem-dependent sGC stimulator YC-1, a synthetic benzylindazole derivative, was described in 1978. The characterisation 20 years later demonstrated that as well as increasing sGC activity, YC-1 acted in synergy with NO to stimulate sGC. However, YC-1 was a relatively weak vasodilator and had side effects. Therefore, the search began for novel indazole compounds that were more potent and more specific sGC stimulators. The result was the identification of BAY 41-2272 and BAY 41-8543. Both compounds were tested in various preclinical studies on different animal models and appeared to improve systemic arterial oxygenation. To improve the pharmacologic and pharmacokinetic profile an additional 1000 compounds were screened leading to the discovery of riociguat. Riociguat was tested in mouse and rat disease models, where it effectively reduced pulmonary hypertension and reversed the associated right heart hypertrophy and ventricular remodelling.
Several clinical trials have been undertaken to investigate and evaluate diverse aspects of riociguat and some of them are still ongoing

Riociguat (BAY 63-2521), N-[4,6-Diamino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]-N-methylcarbamic acid methyl ester, is a soluble guanylate cyclase stimulator. Riociguat is under investigation for the treatment of chronic thromboembolic pulmonary hypertension, and pulmonary arterial hypertension. Michelakis et al., Eur. Respir. J., 2009, 33(4), 717-21; Ghofrani et al., Eur. Respir. Rev., 2009, 18(111), 35-41; Mittendorf et al., ChemMedChem,2009, 4, 853-65; and WO 2006037491.
Riociguat has also shown promise in treating hypertension, angina pectoris, heart failure, atherosclerosis, thromboembolism, platelet activation, asthma, bronchoconstriction, chronic obstructive pulmonary disease, pulmonary vasoconstriction, acute respiratory distress syndrome, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, cerebrovascular disorders, cerebrovasospasm, transient ischemic attacks, stroke, migraine, migraine with vertigo, genitourinary disorders, benign prostate syndrome, benign prostate hyperplasia, benign prostate enlargement, urinary incontinence, bladder outlet obstruction, neurogenic bladder syndrome, pelvic pain, prostate hypertrophy, sexual dysfunction,
Raynaud's phenomenon, increased cellular proliferation, endothelial dysfunction, erectile dysfunction wounds, scarring, fibrotic disorders, renal disorders, renal failure, renal hypertension, idiopathic pulmonary fibrosis, non-alcoholic hepatosteatosis, hepatitis, diabetic nephropathy, hypercholesterolemia, osteoporosis, optic nerve damage, and glaucoma.
Gur et al., Curr. Pharm. Design., 2010, 16, 1619-33; WO 2010081647; WO 2008138483; WO 2007009607; US 20070225299; US 20090215769; US 20090221573; and U.S. Pat. No. 7,173,037.
Adverse effects associated with riociguat include headache, nasal congestion, flushing, overheating sensation, orthostatic hypotension, and heart palpitations.
Subscribe to:
Comments (Atom)
