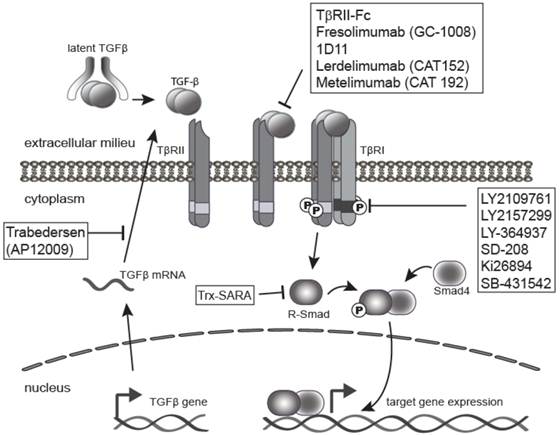
Fresolimumab
GC 1008, GC1008
UNII-375142VBIA
cas 948564-73-6
Structure
- immunoglobulin G4, anti-(human transforming growth factors beta-1, beta-2 (G-TSF or cetermin) and beta-3), human monoclonal GC-1008 γ4 heavy chain (134-215′)-disulfide with human monoclonal GC-1008 κ light chain, dimer (226-226”:229-229”)-bisdisulfide
- immunoglobulin G4, anti-(transforming growth factor β) (human monoclonal GC-1008 heavy chain), disulfide with human monoclonal GC-1008 light chain, dimer
An anti-TGF-beta antibody in phase I clinical trials (2011) for treatment-resistant primary focal segmental glomerulosclerosis.
A pan-specific, recombinant, fully human monoclonal antibody directed against human transforming growth factor (TGF) -beta 1, 2 and 3 with potential antineoplastic activity. Fresolimumab binds to and inhibits the activity of all isoforms of TGF-beta, which may result in the inhibition of tumor cell growth, angiogenesis, and migration. TGF-beta, a cytokine often over-expressed in various malignancies, may play an important role in promoting the growth, progression, and migration of tumor cells.

Fresolimumab (GC1008) is a human monoclonal antibody[1] and an immunomodulator. It is intended for the treatment of idiopathic pulmonary fibrosis (IPF), focal segmental glomerulosclerosis, and cancer[2][3] (kidney cancer and melanoma).
It binds to and inhibits all isoforms of the protein transforming growth factor beta (TGF-β).[2]
History
Fresolimumab was discovered by Cambridge Antibody Technology (CAT) scientists[4] and was one of a pair of candidate drugs that were identified for the treatment of the fatal condition scleroderma. CAT chose to co-develop the two drugs metelimumab (CAT-192) and fresolimumab with Genzyme. During early development, around 2004, CAT decided to drop development of metelimumab in favour of fresolimumab.[5]In February 2011 Sanofi-Aventis agreed to buy Genzyme for US$ 20.1 billion.[6]
As of June 2011 the drug was being tested in humans (clinical trials) against IPF, renal disease, and cancer.[7][8] On 13 August 2012, Genzyme applied to begin a Phase 2 clinical trial in primary focal segmental glomerulosclerosis[9] comparing fresolimumab versus placebo.
As of July 2014, Sanofi-Aventis continue to list fresolimumab in their research and development portfolio under Phase II development.[10]

References
1 WHO Drug Information
2 National Cancer Institute: Fresolimumab
- 3 Statement On A Nonproprietary Name Adopted By The USAN Council – Fresolimumab
- 4 Grütter, Christian; Wilkinson, Trevor; Turner, Richard; Podichetty, Sadhana; Finch, Donna; McCourt, Matthew; Loning, Scott; Jermutus, Lutz; Grütter, Markus G. (2008-12-23). “A cytokine-neutralizing antibody as a structural mimetic of 2 receptor interactions”. Proceedings of the National Academy of Sciences 105 (51): 20251–20256. doi:10.1073/pnas.0807200106. ISSN 0027-8424. PMC 2600578. PMID 19073914.
- 5 http://www.independent.co.uk/news/business/news/cat-may-abandon-skin-drug-after-trial-results-disappoint-569445.html
- 6 http://www.bbc.co.uk/news/business-12477750
- 7 http://www.genengnews.com/gen-news-highlights/scientists-trigger-white-fat-to-become-brown-fat-like-to-treat-obesty-and-type-2-diabetes/81245389/
- 8 Clinicaltrials.gov for Fresolimumab
- 9 http://clinicaltrials.gov/show/NCT01665391
- 10 http://en.sanofi.com/rd/rd_portfolio/rd_portfolio.aspx
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Human |
| Target | TGF beta 1, 2 and 3 |
| Clinical data | |
| Legal status |
|
| Identifiers | |
| CAS Number | 948564-73-6 |
| ATC code | None |
| ChemSpider | none |
| KEGG | D09620 |
| Chemical data | |
| Formula | C6392H9926N1698O2026S44 |
| Molar mass | 144.4 kDa |