Drug to ‘cure’ cravings-compound found in the bark of an African bush may hold clues to the development of drugs for reversing a host of addictive behaviours from drug
(–)-18-Methoxycoronaridine (18-MC)
A compound found in the bark of an African bush may hold clues to the development of drugs for reversing a host of addictive behaviours from drug and alcohol abuse and even smoking and compulsive over-eating, scientists reported at the BIO convention in Chicago, US, in April 2013.
read all at
 | |
|---|---|
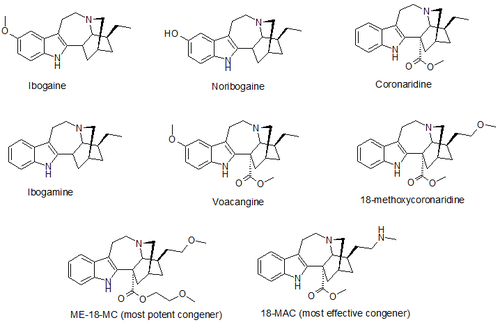
(–)-18-Methoxycoronaridine (18-MC) is a derivative of ibogaine invented in 1996 by the research team around the pharmacologistStanley D. Glick from the Albany Medical College and the chemist Martin E. Kuehne from the University of Vermont. In animal studies it has proved to be effective at reducing self-administration of morphine, cocaine, methamphetamine, nicotine and sucrose. 18-MC is a selective α3β4 nicotinic antagonist and, in contrast to ibogaine, has no affinity at the α4β2 subtype nor at NMDA-channels nor at the serotonin transporter, and has significantly reduced affinity for sodium channels and for the σ receptor, but retains modest affinity for the μ and κ opioid receptors. The sites of action in the brain include the medial habenula, interpeduncular nucleus, dorsolateral tegmentum and basolateral amygdala. It has also been shown to produce anorectic effects in obese rats, most likely due to the same actions on the reward system which underlie its anti-addictive effects against drug addiction.
18-MC has not yet been tested in humans. In 2002 the research team started trying to raise funds for human trials, but were unable to secure the estimated $5 million needed. Efforts to raise funds for future trials are still ongoing. In January 2010, Obiter Research, a chemical manufacturer in Champaign, Illinois, signed a patent license with Albany Medical College and the University of Vermont allowing them the right to synthesize and market 18-MC and other congeners.
A number of derivatives of 18-MC have also been developed, with several of them being superior to 18-MC itself, the methoxyethyl congener ME-18-MC being more potent than 18-MC but with similar efficacy, and the methylamino analogue 18-MAC being more effective than 18-MC but with around the same potency. These compounds were also found to act as selective α3β4 nicotinic acetylcholine antagonists, with little or no effect on NMDA receptors.
BY WORLD DRUG TRACKER