KEBUZONE…….An antirheumatic agent.
Kebuzone (or ketophenylbutazone) is a non-steroidal anti-inflammatory drug.
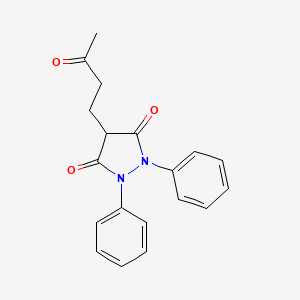
Structural formula
4-(3-oxobutyl)-1,2-diphenylpyrazolidine-3,5-dione
4-(3-Oxobutyl)-1,2-diphenyl-3,5-pyrazolidinedione
Additional Names: 1,2-diphenyl-4-(g-ketobutyl)-3,5-pyrazolidinedione; 1,2-diphenyl-4-(3¢-oxobutyl)-3,5-dioxopyrazolidine; ketophenylbutazone; KPB
Trademarks: Chebutan; Chepirol; Chetazolidin (Zeria); Chetil; Copirene; Ketason; Ketazone (Beytout); Pecnon (Sanken); Phloguron (Steiner); Recheton
MF: C19H18N2O3
MW: 322.36
Percent Comp: C 70.79%, H 5.63%, N 8.69%, O 14.89%
Properties: Crystals, mp 115.5-116.5° or 127.5-128.5° depending on cryst form.
Melting point: mp 115.5-116.5° or 127.5-128.5° depending on cryst form
Therap-Cat: Antirheumatic.
- BRN 0308507
- Chebutan
- Chepirol
- Chetazolidin
- Chetil
- Copirene
- EINECS 212-715-7
- Hichillos
- Kebuzone
- Kebuzonum
- Kebuzonum [INN-Latin]
- Keobutane-jade
- Ketason
- Ketazone
- Ketophenylbutazone
- Ketophenylbutazonum
- KPB
- Pecnon
- Quebuzona
- Quebuzona [INN-Spanish]
- Recheton
- UNII-4VD83UL6Y6
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions.They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects.
UV – range
IR – spectrum
Reference
- UV and IR Spectra. H.-W. Dibbern, R.M. Muller, E. Wirbitzki, 2002 ECV
- NIST/EPA/NIH Mass Spectral Library 2008
- Handbook of Organic Compounds. NIR, IR, Raman, and UV-Vis Spectra Featuring Polymers and Surfactants, Jr., Jerry Workman. Academic Press, 2000.
- Handbook of ultraviolet and visible absorption spectra of organic compounds, K. Hirayama. Plenum Press Data Division, 1967.
Brief background information
| SALT | ATC | FORMULA | MM | CAS |
|---|---|---|---|---|
| - | M01AA06 | C 19 H 18 N 2 O 3 | 322.36 g / mol | 853-34-9 |
| 4-(3-oxobutyl)-1,2-di(phenyl)pyrazolidine-3,5-dione | |
| CLINICAL DATA | |
|---|---|
| LEGAL STATUS |
?
|
| IDENTIFIERS | |
| CAS NUMBER | 853-34-9 |
| ATC CODE | M01AA06 |
| PUBCHEM | CID 3824 |
| CHEMSPIDER | 3692 |
| UNII | 4VD83UL6Y6 |
| KEGG | D01567 |
| CHEBI | CHEBI:31749 |
| CHEMICAL DATA | |
| FORMULA | C19H18N2O3 |
| MOL. MASS | 322.35782 g/mol |
Application
- anti-inflammatory
- antirheumatic
- Synthesis pathway
Trade names
| COUNTRY | TRADE NAME | MANUFACTURER |
|---|---|---|
| Germany | Kebuzon | Steiner |
| France | Ketazon | Beytout |
| Italy | Chetopir | Sarm |
| Ukraine | no | no |
Formulations
- ampoules of 1 g / 5 ml;
- 250 mg capsule
Reference
- Synthesis of a)
- Denss, R. et al .: Helv. Chim. Acta (HCACAV) 40, 402 (1957).
- material:
- Kühn, M .: J. Prakt. Chem. (JPCEAO) 156 (II), 103 (1940).
- Synthesis b)
- AT 198 263 (Synfarma; appl. 1955).
References: Prepn: Deuss et al., US 2910481 (1959 to Geigy).
Review of pharmacology: Horakova et al.,Pharmacotherapeutica 1950-1959, 335-350 (1963), C.A. 60, 6072g (1964).
Metabolism: Nemecek et al., Arzneim.-Forsch. 16,1339 (1966); Queisnerova, Nemecek,Cesk. Farm. 20, 55 (1971), C.A. 75, 47077u (1971).
Herrenknecht, Christine; Guernet-Nivaud, Elisabeth; Lafont, Olivier; Guernet, Michel; Gueutin, Claire
Canadian Journal of Chemistry, 1988 , v. 66, pg. 1199 – 1202
Canadian Journal of Chemistry, 1988 , v. 66, pg. 1199 – 1202
Cizmarik; Lycka
Pharmazie, 1988 , v. 43, 11 pg. 794 – 795
Pharmazie, 1988 , v. 43, 11 pg. 794 – 795
Gueutin-Pelinard, Claire; Nivaud, Elisabeth; Boucly, Patrick; Guernet, Michel
Canadian Journal of Chemistry, 1981 , v. 59, pg. 759 – 762
Canadian Journal of Chemistry, 1981 , v. 59, pg. 759 – 762
Denss et al.
Helvetica Chimica Acta, 1957 , v. 40, pg. 402,406
Helvetica Chimica Acta, 1957 , v. 40, pg. 402,406
Patent: CS124279 , 1965 ;Chem.Abstr., 1968 , v. 69, 52134r
SPOFA; United Pharmaceutical Work Patent: FR1500627 , 1965 ;Chem.Abstr., 1968 , v. 69, 96715k
Nippon Shinyaju Co., Ltd. Patent: US5811547 A1, 1998 ;
Fisnerova,L. et al. Collection of Czechoslovak Chemical Communications, 1974 , v. 39, pg. 624 – 633












