CANTHARIDIN
(1R,2S,6R,7S)-2,6-Dimethyl-4,10-dioxatricyclo[5.2.1.02,6]decane-3,5-di
Spanish fly is one of the oldest, most legendary aphrodisiacs. It’s made from the crushed bodies of insects, which oddly enough aren’t flies and don’t come from Spain but are beetles in the familyMeloidae, called blister beetles that live worldwide. The French soldiers denied using Spanish fly but did admit to supplementing their military rations eating frogs from a local stream. Was there a link between frogs and Spanish fly? The smart doctor went to the water’s edge and found frogs busily devouring a swarm of emerald coloured beetles. He surmised that whatever noxious substance is found inside these 'Spanish flies”' also hung around inside the frogs giving the troops more than they bargained for.
Cantharidin, a type of terpenoid, is a chemical compound secreted by many species of blister beetle, and most notably by theSpanish fly, Lytta vesicatoria. The false blister beetles, cardinal beetles and soldier beetles also produce cantharidin. It is apoisonous substance, acting as a blister agent, and can cause severe chemical burns, but these same properties make it effective as a topical medication.
Cantharidin was first isolated in 1810 by Pierre Robiquet, a French chemist then living in Paris, from Lytta vesicatoria. Robiquet demonstrated that cantharidin was the actual principle responsible for the aggressively blistering properties of the coating of the eggs of that insect, and established that cantharidin had definite toxic properties comparable in degree to those of the most virulent poisons known in the 19th century, such as strychnine. It is an odorless and colorless solid at room temperature. It is secreted by the male blister beetle and given to the female as a copulatory gift during mating. Afterwards the female beetle will cover its eggs with it as a defense against predators. The complete mechanism of the biosynthesis of cantharidin is currently unknown.
The level of cantharidin in blister beetles can be quite variable: Among blister beetles of the genus Epicauta in Colorado, E. pennsylvanica contain approximately 0.2 mg, E. maculata contain 0.7 mg, and E. immaculata contain 4.8 mg per beetle; males also contain higher levels than females.
The active ingredient in Spanish fly is a bicyclic terpenoid called cantharidin, an odourless, colourless solid at room temperature. Its effects on the human body have been known for millennia. The ancient Greek physician, Hippocrates, prescribed ground blister beetles as a treatment for dropsy. Traditional Chinese medicine uses blister beetle to treat piles, ulcers, and rabies. Whether any of these treatments actually work remains unclear. But there’s no doubt that Spanish fly is powerful stuff. Let a blister beetle scuttle across your hand and you better hope it’s in a good mood. When angry or alarmed, they emit cantharidin drops that will bring you out in blisters.
Cantharidin is absorbed by lipid layers in the skin’s epidermis, disrupting transmembrane proteins that hold cells together resulting in blistering and lesions. Since the 1950s it’s been used to treat warts and another viral skin condition called molluscum contagiosum but only under strict medical supervision. Don’t go rubbing yourself with beetles.
More dangerous is the effect of eating blister beetles. When consumed, cantharidin inflames the gastrointestinal tract and, depending on the dose, can completely strip away the stomach lining. As the kidneys try to purge the toxin from the blood it causes swelling in the urinary tract in what can appear to be arousal but is far from it: swallow two or three blister beetles and they could kill you. Cantharidin is about as toxic as cyanide and strychnine and there’s no known antidote. Notorious French aristocrat, the Marquis de Sade, got in trouble for giving prostitutes a box of chocolates laced with Spanish fly. The women survived but de Sade was sentenced to death for attempted murder.

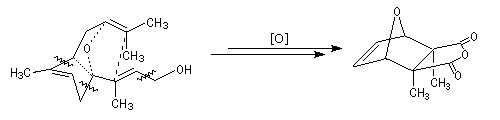
The cantharidin was first isolated by Robiquet, a French chemist in 1810. It has an important role in the ecology of different kinds of insects that use or produce it as a defense ability to preserve their eggs from predators[1]. In the ancient world, the dried spanish flies had the reputation of aphrodisiac virtues [27]. In fact, these supposed properties are not attested neither in theory nor in experimentation. The cantharidin seems to be a dangerous substance with the same toxicity as the most violent poisonus like strychnin.
 | Two insect-families belonging to beetles produce this substance : theMeloidae and the Oedemeridae. The first family groups together thousands species in which the Epicauta are the most widespread. Their most known representation is named Lytta Vesicatoria or Spanish fly[20]. The opposite photography represents an example of this insect. |
The cantharidin molecule
At the usual temperature, the cantharidin is an odorless and colorless solid. Its formula is C10H12O4 and its melting point is 218 °C.
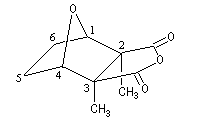 The cantharidin or 2, 3-dimethyl-7-oxabicyclo[2, 2, 1]heptane-2, 3-dicarboxylic anhydrid represented on the picture shown above, is an achiral compound. It has a plane of symmetry that goes through the middles of the bonds C2C3 and C5C6. It's a meso compound (2S, 3R). |
There is an other molecule with a similar structure : the palasonin. This molecule is produced by a tree butea frondosa that grows in the Himalaya. Contrary to the cantharidin, the palasonin is chiral and exists with two enantiomeric forms. The natural compound represented below is (-)-palasonin.
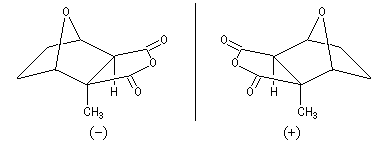 |
Biosynthesis
Among the insects, the cantharidin is produced by the male and transfered to the female during the mating. The female will cover its eggs with this compound in a defense purpose. The complete mechanism of the biosynthesis is not known at the moment. But it is quite sure that it implicates reactions with as first compound a terpenic alcohol. A proof can be given with mass spectroscopy using the 14C, 3H, 18O isotopes [9].
Among the insects, the cantharidin is produced by the male and transfered to the female during the mating. The female will cover its eggs with this compound in a defense purpose. The complete mechanism of the biosynthesis is not known at the moment. But it is quite sure that it implicates reactions with as first compound a terpenic alcohol. A proof can be given with mass spectroscopy using the 14C, 3H, 18O isotopes [9].
The farnesol represented on the picture is the (E, E)-3, 7, 11-triméthyldodeca-2, 6, 10-triene-1-ol. This is a sesquiterpenic alcohol. It is an intermediate in the biosynthesis of isoprenoïds.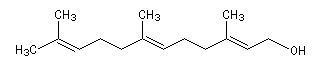 The farnesol is quite abundant in the nature. A lot of natural essences, like Ylang-ylang ones, contain this molecule. |
The following diagram shows some cleavages and insertion of oxygen in a specific conformation of the farnesol that will lead to the cantharidin [20].
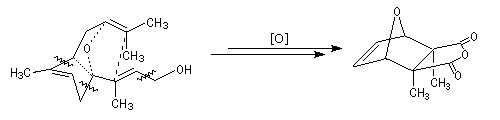
Mode of action of the cantharidin
The cantharidin inhibits the proteinphospatase 2A (PP2A), an enzyme that operates in the metabolism of glycogen [23].
The cantharidin inhibits the proteinphospatase 2A (PP2A), an enzyme that operates in the metabolism of glycogen [23].
First attemps for the synthesis of the cantharidin
Von Bruchhausen, a german chimist, first attempted the synthesis of the cantharidin [2]. It was based on the following retrosynthetic analysis.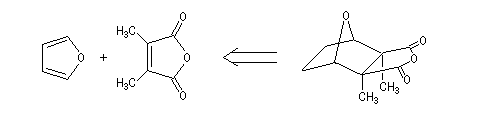
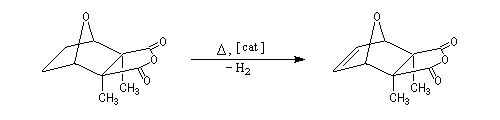
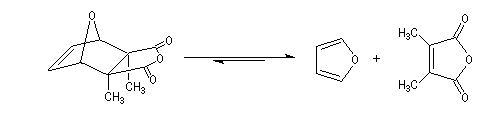
A more deepened study shows that the instability of the product is due to the repulsion between the methyl groups carried by C2 and C3 and also to the repulsion between the hydrogen carried by C5 et C6 (the covering between the Van der Waals spheres is visible with the "spacefill" representation of the molecular model of the cantharidin).
Synthesis of the cantharidin
The Stork synthesis
The first effective synthesis of the cantharidin was made in 1951 by the American chemist G. Stork (born in Belgium) [4] and [5]. The synthesis is completly linear and only uses classical reactions. It's a typical total synthesis of natural product dating from this period.
The first effective synthesis of the cantharidin was made in 1951 by the American chemist G. Stork (born in Belgium) [4] and [5]. The synthesis is completly linear and only uses classical reactions. It's a typical total synthesis of natural product dating from this period.
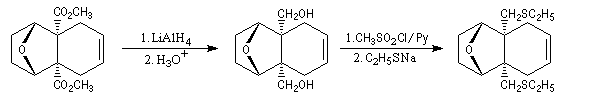
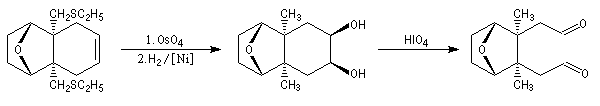
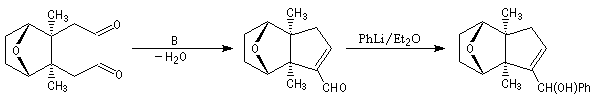
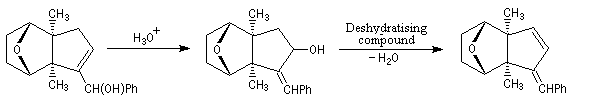
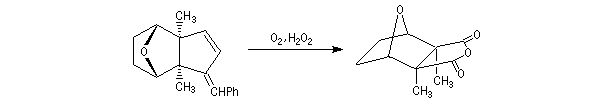
The Schenk synthesis
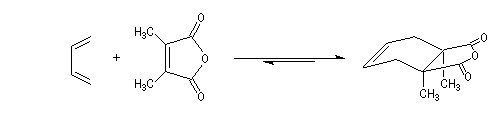
The Schenk's synthesis first uses a Diels-Alder reaction but here, the repulsions between the methyl groups don't exist and the equilibrium is favourable to the product [6].
The Schenk's synthesis first uses a Diels-Alder reaction but here, the repulsions between the methyl groups don't exist and the equilibrium is favourable to the product [6].
Conjugated double bonds are made with the addition of Br2 on the ethylenic bond following by a double elimination with DBU.
DBU means 1,8-diazabicyclo[5.4.0]undec-7-ene. It's a bulky base that permits elimination.
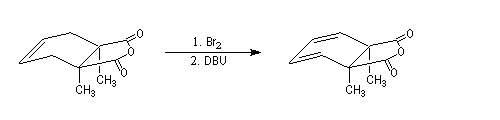
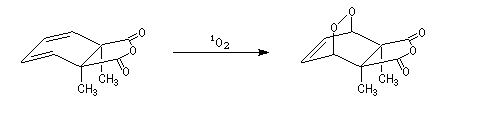
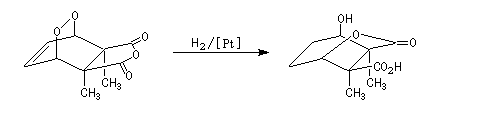
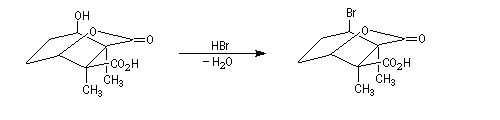
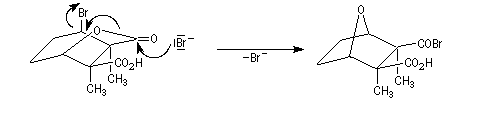
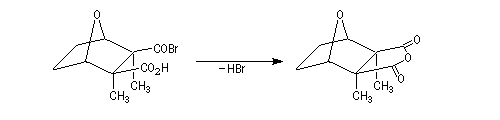
The Dauben's synthesis
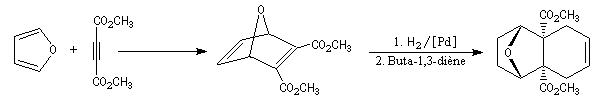
Dauben is an American chemist in Berkeley University. He uses high pressure to realize a lot of synthesis. The Diels-Alder's reaction is realizable thanks to a pressure of 15 kbar. And then the synthesis of cantharidin necessitates only two steps [7], [8].
Dauben is an American chemist in Berkeley University. He uses high pressure to realize a lot of synthesis. The Diels-Alder's reaction is realizable thanks to a pressure of 15 kbar. And then the synthesis of cantharidin necessitates only two steps [7], [8].
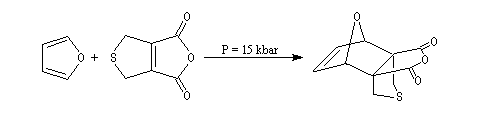
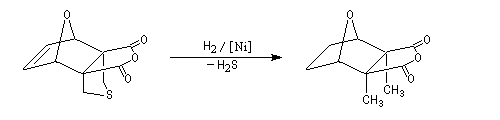
Bibliography
[1] Robiquet. M. Ann. Chim. 1810, 76, 302-307.
[2] von Bruchhausen, F.; Bersch, II. W. Arch. Pharm. Ber. Disch. Phurm. Ges. 1928, 266, 697-702.
[3] Diels. O.; Alder, K. Ber. 1929, 62, 554-562.
[4] Stork, G.; and al.J. Am. Chem. Soc. 1951, 73, 4501.
[5] Stork, G.; van Tamelen, E. E.; Friedman, L. I.; Burgstahler, A. W. J. Am. Chem. Soc. 1953, 75, 384.
[6] Schenck, G.; Wirtz, R. Naturwissenshaften 1953, 40, 531.
[7] Dauben, W. G.; Kessel, C. R.; Takemura, K. H. J. Am. Chem. Soc. 1980, 102, 6893-6894.
[8] Dauben, W. G.; Krabbenhoft, II. O. J. Am. Chem. Soc. 1976, 98, 1992-1993.
[9] McCormick, J. P.; Carrel, J. E.; Doom, J. P. J. Am. Chem. Soc. 1986, 108, 8071-8074.
[10] Sierra, J. R.; Woggon, W. D.; Schmid, H. Experientia 1976, 32, 142-144.
[1] Robiquet. M. Ann. Chim. 1810, 76, 302-307.
[2] von Bruchhausen, F.; Bersch, II. W. Arch. Pharm. Ber. Disch. Phurm. Ges. 1928, 266, 697-702.
[3] Diels. O.; Alder, K. Ber. 1929, 62, 554-562.
[4] Stork, G.; and al.J. Am. Chem. Soc. 1951, 73, 4501.
[5] Stork, G.; van Tamelen, E. E.; Friedman, L. I.; Burgstahler, A. W. J. Am. Chem. Soc. 1953, 75, 384.
[6] Schenck, G.; Wirtz, R. Naturwissenshaften 1953, 40, 531.
[7] Dauben, W. G.; Kessel, C. R.; Takemura, K. H. J. Am. Chem. Soc. 1980, 102, 6893-6894.
[8] Dauben, W. G.; Krabbenhoft, II. O. J. Am. Chem. Soc. 1976, 98, 1992-1993.
[9] McCormick, J. P.; Carrel, J. E.; Doom, J. P. J. Am. Chem. Soc. 1986, 108, 8071-8074.
[10] Sierra, J. R.; Woggon, W. D.; Schmid, H. Experientia 1976, 32, 142-144.
Links
[20] Ecology and Biosynthesis of Cantharidin and Palasonin
[21] O. Diels and K. Alder the Nobel Prize in Chemistry 1950
[22] Oxygen inorganic photochemistry
[23] Design and synthesis of selective phosphatase inhibitors
[24] Cantharidin : A Historical Overview and Synthetic Approach
[25] How does cantharidine work and what is it used for
[26] The British Pharmaceutical Codex, Cantharis B. P.
[27] Aphrodisiacs their biological basis
[28] Blister beetle intoxication