
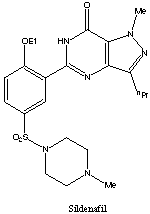
1-[4-ethoxy-3-(6,7-dihydro-1-methyl-
7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)
phenylsulfonyl]-4-methylpiperazine
CAS NO 139755-83-2
Sildenafil citrate, sold as Viagra, Revatio and under various other trade names, is a drug used to treat erectile dysfunction and pulmonary arterial hypertension (PAH). It was originally developed by British scientists and then brought to market by the US-based pharmaceutical company Pfizer.[1]It acts by inhibiting cGMP-specific phosphodiesterase type 5 (PDE5), an enzyme that promotes degradation of cGMP, which regulates blood flow in the penis. Since becoming available in 1998, sildenafil has been the prime treatment for erectile dysfunction; its primary competitors on the market are tadalafil (Cialis) and vardenafil (Levitra)
Erectile dysfunction (or ED), also called male impotence, describes a mans inability to achieve and maintain an erection sufficient for mutually satisfactory sexual intercourse with his partner. By itself, ED is not a disease but more of a signal that something else may be a problem.
Viagra works best if taken 30-60 minutes before sexual activity. Only 1 tablet should be taken per day. It should be taken on an empty stomach. Increasing the dosage beyond the recommended amounts will not improve the response and will only result in greater side effects.
Sildenafil is a vasoactive agent used to treat erectile dysfunction and reduce
symptoms in patients with pulmonary arterial hypertension (PAH).
symptoms in patients with pulmonary arterial hypertension (PAH).
Sildenafil elevates levels of the second messenger, cGMP, by inhibiting its breakdown
via phosphodiesterase type 5 (PDE5). PDE5 is found in particularly high
concentrations in the corpus cavernosum, erectile tissue of the penis.
via phosphodiesterase type 5 (PDE5). PDE5 is found in particularly high
concentrations in the corpus cavernosum, erectile tissue of the penis.
It is also found in the retina and vascular endothelium.
Increased cGMP results in vasodilation which facilitates generation and
maintenance of an erection. The vasodilatory effects of sildenafil also help reduce
symptoms of PAH.
Sildenafil citrate, sold as Viagra, Revatio and under various other trade names,
is adrug used to treat erectile dysfunction and pulmonary arterial
hypertension (PAH).
It was originally developed by British scientists and then brought to market by the
US-basedpharmaceutical company Pfizer.
maintenance of an erection. The vasodilatory effects of sildenafil also help reduce
symptoms of PAH.
Sildenafil citrate, sold as Viagra, Revatio and under various other trade names,
is adrug used to treat erectile dysfunction and pulmonary arterial
hypertension (PAH).
It was originally developed by British scientists and then brought to market by the
US-basedpharmaceutical company Pfizer.

It acts by inhibiting cGMP-specific phosphodiesterase type 5, an enzyme that promotes degradation of cGMP, which regulates blood flow in thepenis.
Since becoming available in 1998, sildenafil has been the prime treatment for erectile dysfunction; its primary competitors on the market are tadalafil (Cialis) and vardenafil (Levitra).
VIAGRA® (sildenafil citrate) , an oral therapy for erectile dysfunction, is the citrate salt of sildenafil, a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5).
Sildenafil citrate is designated chemically as 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine citrate and has the following structural formula:
 |
Sildenafil citrate is a white to off-white crystalline powder with a solubility of 3.5 mg/mL in water and a molecular weight of 666.7. VIAGRA (sildenafil citrate) is formulated as blue, film-coated rounded-diamond-shaped tablets equivalent to 25 mg, 50 mg and 100 mg of sildenafil for oral administration. In addition to the active ingredient, sildenafil citrate, each tablet contains the following inactive ingredients: microcrystalline cellulose, anhydrous dibasic calcium phosphate, croscarmellose sodium, magnesium stearate, hypromellose, titanium dioxide, lactose, triacetin, and FD & C Blue #2 aluminum lake.
Target is cyclic guanosine monophosphate phosphodiesterase type 5 receptor.
Fluforma is a company which found the genome explorations and target identification
through affymetrix microarrays.
through affymetrix microarrays.
High throughput functional assays of Si Rna’s inhibit the expression of target,
chromatographic techniques, spectrophotometric methods, adsorptive
stripping voltametry.
chromatographic techniques, spectrophotometric methods, adsorptive
stripping voltametry.
Gingel et.al showed that a 50mg dose given daily for 28 days consecutively
improved errections in almost 90% of patients.
Structural validation techniques are NMR technique, Infrared spectrum, HPLC, Mass spectroscopy and Liquid chromatography.improved errections in almost 90% of patients.

The chemical name of sildenafil is 5-[2-ethoxy-5-(4-methylpiperazin-1-ylsulfonyl)phenyl]-1- methyl-3-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one and its formula is C22H30N6O4S. The melting point of sildenafil is 189-190oC. Its solubility is 3.5 mg/mL in water. | |||||||||||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||||||||||
The 1H NMR data of sildenafil is given below. The abbreviations used are s for singlet, d for doublet, t for triplet and q for quartet. The chemical shifts are given in ppm (parts per million) and are followed by the number of Hydrogens the peaks account for:
| |||||||||||||||||||||||||||||||||||||
 Viagra aims at inhibiting the enzyme phosphodiesterase PDE5. It must therefore have a structure that is similar in some places to the substrate. However, there are many other constraints as there are several different types of PDE enzymes which are found in different parts of the body. Of the 7 types of PDE, three selectively hydrolyse cGMP relative to cAMP. PDE5 itself can be found in several parts of the body : the lungs, platelets and various forms of smooth muscle. Selectivety was a very important factor in the research for an inhibitor of PDE5. The research on a molecule to inhibit PDE type 5 started with the molecule Zaprinast (1) which is shown on the right. The research established that derivatives of pyrazolo[4,3-d]pyrimidin-7-one (2) gave more potent cGMP PDE5 inhibition. The studies carried out compared many molecules by changing the substituents on them and comparing the inhibitory data of these molecules. To establish the selectivity of the compound, the compounds were also screened against the other widespread cGMP enzymes, PDE1 which was isolated from rat liver and PDE3 which was isolated from rat platelet. Further studies showed that sildenafil was the best PDE5 inhibitor. The different functional groups on the molecule were established by comparing the affinities of the molecules to the different PDE enzymes and establishing the selectivity of each compound made. Of course, high selectivity for PDE type 5 was looked for. Viagra mimics the guanosine base of cGMP and the extension of 3-substituent fills a space in the enzyme active site occupied by ribose. Substituents on the 5'-position of the phenyl ring reproduce the role of the phosphate bonding. To improve the solubility of the drug, polar substituents were added which gave compounds with a lower lipophilicity. This was found to also increase the enzyme affinity. Sidenafil gave an excellent combination of enzyme inhibitory potency, selectivity, solubility and in vivo characteristics. | 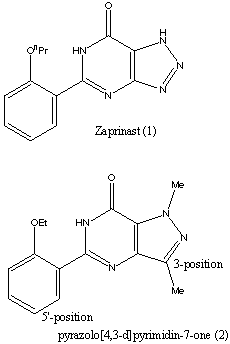 | ||||||||||||||||||||||||||||||||||||
It is interesting to compare the structure of sidenafil with that of cGMP (cyclic guanosine monophosphate), which is the molecule that usually interacts with PDE5. The drug that was being developed by the researchers had to have some similarity so that it could bind to the active site of the enzyme, PDE5. The 2 dimensional structure of cGMP is given above the 3 dimensional picture, however the latter can be used to view the molecule in 2D as well, with any orientation of the molecule. The first figure also shows that cGMP and pyrazolo[4,3-d]pyrimidin-7-one, which is one of the parent molecules of sildenafil, have a similar size, shape and dipole moment. These were characteristics that were looked for in a molecule that would inhibit PDE5. The following molecule is cGMP :  | |||||||||||||||||||||||||||||||||||||
Sildenafil is the active ingredient in Viagra ™ and it is chemically designated as 5-[2- ethoxy-5 -(4-methyl)piperazine- 1 -yl-sulfonyl)phenyl]- 1 -methyl-3-n-propyl- 1 ,6-dihydro- 7H-pyrazolo[4,3-d]pyrimidin-7-one, and the following chemical structure V:
V
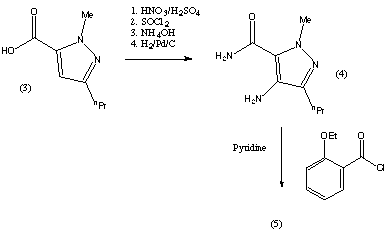
Sildenafil is originally disclosed in US Patent No. 5,250,534 and it has been found to be particularly useful in the treatment of inter-alia male erectile dysfunction. Multi step syntheses for the production of Sildenafil are disclosed in US'534 with a yield of 27%.
An improved process for its preparation is described in a later application i.e. US Patent No. 5,955,611 which consisting the preparation of 5-chlorosulfonyl-2-ethoxybenzoic acid and converting it into 2-ethoxy-5-(4-methylpiperazin-l-ylsulfonayl)benzoic acid. The acid is then condensed with 4-amino-l-methyl-3-n-propylpyrazole-5-carboxyamide in the presence of N,N'-carbonyldiimidazole, and the resulting 4-[2-ethoxy-5-(4- methylpiperzin-l-ylsulfonyl)benzamido]-l-methyl-3-n-propylpyrazolo-5-carboxyamide is cyclized in an alkaline, neutral or acid solution to yield Sildenafil with about 47% yield.
Patent application number WO 2001/22918 describes the process for the preparation of Sildenafil wherein the process involves the reaction step between 2-ethoxy-5-(4-methyl- piperazine- 1 -sulfonyl)benzaldehyde and 4-amino- 1 -methyl-S-n-propyl-pyrazole-S- carboxamide to form an intermediate which is further cyclised to form Sildenafil.
Patents/ patent applications US6204383, US20030069422, US20030144530, US 20040106796, US 20040110948, EP 0812845, EP 1002798, EP 1077214, WO 01/19827, WO 04/31134 also discloses several processes for the preparation of Sildenafil citrate.
Sildenafil citrate is a white to off-white crystalline powder with a solubility of 3.5 mg/mL in water and a molecular weight of 666.7. Sildenafil citrate has most recently been utilized as the basis for an oral therapy for erectile dysfunction and has been marketed by Pfizer Labs under the trademark Viagra®. Publications relating to benign visual side-effects (e.g., blue-shift in vision, light-sensitivity, and blurring noted to occur in some patients) of sildenafil prompted the FDA to insist on product insert warnings.
Sildenafil citrate is a white to off-white crystalline powder with a solubility of 3.5 mg/mL in water and a molecular weight of 666.7. Sildenafil citrate has most recently been utilized as the basis for an oral therapy for erectile dysfunction and has been marketed by Pfizer Labs under the trademark Viagra®. Publications relating to benign visual side-effects (e.g., blue-shift in vision, light-sensitivity, and blurring noted to occur in some patients) of sildenafil prompted the FDA to insist on product insert warnings.
Sexual dysfunction
The primary indication of sildenafil is treatment of erectile dysfunction (inability to sustain a satisfactory erection to complete intercourse). Its use is now standard treatment for erectile dysfunction in all settings, including diabetes.[2]
People on antidepressants may experience sexual dysfunction, either as a result of their illness or as a result of their treatment. A 2003 study showed that sildenafil improved sexual function in men in this situation.[3] Following up reports from 1999,[4] the same researchers found that sildenafil improved sexual function in female patients on antidepressants as well.[5]
Pulmonary hypertension
As well as erectile dysfunction, sildenafil citrate is also effective in the rare disease pulmonary arterial hypertension (PAH). It relaxes the arterial wall, leading to decreased pulmonary arterial resistance and pressure. This, in turn, reduces the workload of the right ventricle of the heart and improves symptoms of right-sided heart failure. Because PDE5 is primarily distributed within the arterial wall smooth muscle of the lungs and penis, sildenafil acts selectively in both these areas without inducing vasodilation in other areas of the body. Pfizer submitted an additional registration for sildenafil to the United States Food and Drug Administration (FDA), and sildenafil was approved for this indication in June 2005. The preparation is named Revatio, to avoid confusion with Viagra, and the 20 milligram tablets are white and round. Sildenafil joins bosentan and prostacyclin-based therapies for this condition.[6]
|

SCHEME2

Example 1
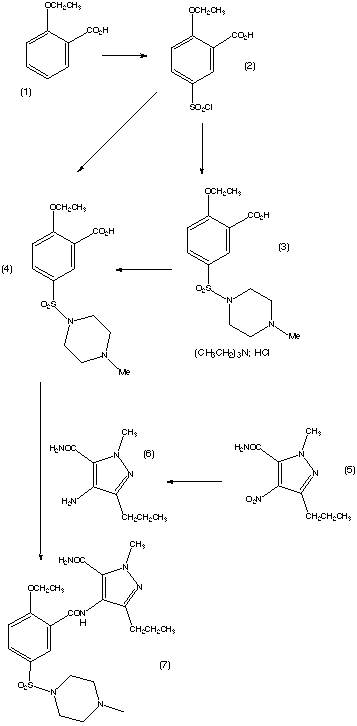
Preparation of 2- hydroxy-5-(4 methyl)-l-piperazinyl sulphonyl) benzoic acid Step-1: Preparation of 5-Chlorosulfonyl-2-hydroxy benzoic acid
To the chilled chlorosulfonic acid (1012 g), salicylic acid (200 g) was added at 0-50C over a period of 1 hour 40 min. The temperature of the reaction mixture was maintained at 20-250C for 2 hrs. Then thionyl chloride (172.4 g) was added over a period of 15 min and maintained for 12 hrs. The product formed was poured onto ice and maintained for lhr. The product was filtered and washed with DM water to get 5-Chlorosulfonyl-2- hydroxy benzoic acid.
Step-2: Preparation of 2-hydroxy-5-(4-methyI)-l-piperazinylsulphonyl)benzoic acid
5-Chlorosulfonyl-2-hydroxy benzoic acid (40Og) obtained in step 1 was dissolved in acetone (1200 ml) and cooled to 5-100C. To this clear solution N-methyl piperazine (254 g) was added and maintained for 2 hrs. The product formed was filtered, washed with water and purified in methanol to get 308 g of the titled compound.
NMR Data:
1H-NMR (300 MHz in DMSO-d6): δ 2.78 (3H, s), 3.17 (8H, brs), 6.85(1H, d, J = 8.7),
7.52 - 7.56 (IH, dd, J=8.7, 2.7), 7.95 (IH, d, J = 2.7)
13C-NMR (75 MHz in DMSO-d6): δ 41.98, 43.36, 51.60, 117.58, 118.33, 119.46,
130.28, 132.01, 167.63, 170.35.
Melting point: 268-2720C
Purity by HPLC: 99.4% Example 2
Preparation of 4-[2-hydroxy-5-(4-methyI-l-piperazinyIsulphonyl)benzamido]-l- methyl-3-n-propyl-lH-pyrazole-5-carboxamide
2-Hydroxy-5-(4-methyl-l-piperazinylsulphonyl)benzoic acid (10Og) was dissolved in dichloromethane (500 ml) and triethylamine (50 ml) followed by distillation to get residual mass. The residual mass was dissolved in dichloromethane (1500ml) followed by the addition of 1,3-dicyclohexylcarbodiimide (75.6 g) and 1-hydroxybenzotriazole (45g). The reaction mixture was stirred at 27-280C and then 4-amino-l-methyl-3-n-propyl- pyrazole-5-carboxamide (60.6 g) was added. The reaction mixture was heated to reflux temperature and maintained for 3 hours. Filtered the undissolved material at hot and washed the cake with dichloromethane (200ml). The filtrate was distilled out completely to get residue. Dissloved the residue in methanol (300ml) at 4O0C and then cooled the mass to 27-280C and stirred overnight. Further, cooled the mass to 5-70C and stirred for lhr. Filtered the product and washed the cake with chilled methanol (100ml) and dried to get 130 g of title compound.
NMR Data:
1H-NMR (300 MHz in DMSO-d6): δ 0.87 (3H, t, J = 7.5), 1.53-1.60 (2H, m), 2.39- 2.46(5H, m), 2.72 (4H, brs), 2.96 (4H, brs), 3.17 (3H, s), 3.91 (3H, s), 6.93 (H, d, J = 8.7), 7.57-7.61 (H, dd, J=8.7 & 2.1), NH2-(2H, brs, J =7.69 & 7.72), 8.15 (IH, d, J=2.1) 11.5 (OH, br).
13C-NMR (75 MHz in DMSO-(I6): 613.80, 21.37, 27.45, 44.05, 44.75, 48.60, 52.87, 116.37, 118.06, 119.67, 120.03, 130.64, 132.17, 132.38, 146.16, 160.83, 166.33, 166.89.
Purity by HPLC: 97.5%
Example 3
Preparation of 5-[2-hydroxy-5-(4-methylpiperazinyl-l-yl-sulphonyl)phenyl]-l- methyl -3-n- propyl-l,6-dihydro-7H-pyrazolo-[4,3-d]pyrimidin-7-one Sodium hydroxide (34 g) was added into diethylene glycol (780ml) and then heated to 110-1150C. 4-[2-hydroxy-5-(4-methyl-l-piperazinylsulphonyl)benzamido)-l-methyl-3-n- propyl-lH-pyrazole-5-carboxamide (130 g) obtained from example 2 was added to the above reaction mixture. The reaction mixture was maintained at 125-13O0C for 4-6 hrs. The reaction mixture was cooled to room temperature and then DM water (1300ml) was added slowly over 20min at 250C and maintained at this temperature for 1 hour. Filtered the mass and filtrate pH was adjusted to 6.5-7.5 with dilute hydrochloric acid at room temperature and stirred at room temperature for 2-3hrs. Product was filtered and slurried the cake with excess DM Water followed by purification in methanol to get 91 g of titled compound.
NMR Data:
1H-NMR (300 MHz in DMSO-d6): δ 0.96 (3H, t, J=7.2), 1.71-1.83 (2H, m), 2.41 (3H, s), 2.78-2.83 (6H, m), 2.99 (4H, brs), 4.15 (3H3 s), 6.93 (IH, d, J=8.7), 7.54-7.57 (IH, dd, J=8.7, 2.1), 8.47 (lH, d, J=2.1).
13C-NMR (75MHz in DMSO-d6): 513.84, 21.52, 27.20, 37.80, 43.94, 44.72,- 52.80, 115.97, 119.82, 120.19, 124.48, 128.71, 131.13, 136.46, 143.82, 151.26, 154.05, 167.24.
Purity by HPLC: 97.8%
Example 4
Preparation of 5-[2-ethoxycarbonyloxy-5-(4-methylpiperazin-l-yl-sulfonyI)phenyl]- l-methyI-3n-propyI-l,6-dihydro-7H-pyrazolo-[4,3-d]pyrimidin-7-one
5-[2-hydroxy-5-(4-methylpiperazinyl-l -yl-sulphonyl)phenyl]- 1 -methyl-3 -n- propyl- 1 ,6- dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one (90 g) obtained from example 3 was dissolved in dichloromethane (360 ml) and added triethyl amine (41 ml) at room temperature and stirred for 10 min. The reaction mixture was cooled to 0-50C and followed by the addition of ethyl chloro formate (24ml) over 30 min under nitrogen atmosphere. The temperature of the reaction was raised slowly to 28-3O0C and maintained for 24 hrs. The reaction mixture was cooled to 0-50C and kept it for 1 hr. The product formed was filtered, washed with dichloromethane, dried and purified from methanol (270ml) to obtain 81 g of the title compound.
NMR Data:
1H-NMR (300 MHz in DMSO-d6): δ 0.92 (3H, t, J=7.2), 1.17 (3H, t, J=7.2), 1.68-1.75 (2H, m), 2.16 (3H, s), 3.99 (4H, br), 2.73 (2H, t, J=7.0), 4.12-4.19 (2H, t, J=6.9), 4.15 (3H, s), 7.71 (IH, d, J = 8.7), 7.93-7.97 (IH, dd, J=8.7 & 2.1), 8.01 (IH, d, J=2.0)
13C-NMR (75 MHz in DMSO-d6): 513.47, 13.80, 21.57, 27.03, 37.90, 45.72, 53.49, 65.12, 124.51, 127.65, 130.14, 130.61, 132.82, 137.30, 144.96, 146.51, 151.38, 151.66, 154.36.
Purity by HPLC: 98.6%
Example 5
Preparation of 5-[2-ethoxy-5-(4-methyl piperazine-l-ylsulfonyl)phenyl]-l-methyl-3- n-propyl-1 ,6-dihydro-7H-pyrazolo [4,3-d] pyrimidin-7-one (Sildenafil base)
5-[2-Ethoxycarbonyloxy-5-(4-methylpiperazin-l-yl-sulfonyl)phenyl]-l-methyl-3-n- propyl-l,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one (50g) was dissolved in ethanol (750ml) in an autoclave and then added dicyclohexylcarbodimide (29.8g). The reaction temperature was raised to HO0C with internal pressure of 1.8-4.0 kg/cm and maintained for 6 hours followed by cooling to room temperature. The solvent was distilled off to get the crude Sildenafil base. The base thus obtained was dissolved in dichloromethane (380ml), filtered and filtrate was distilled out completely to get solid material, which is again dissolved in a mixture dichloromethane and isopropyl ether. The crude obtained was recrystallized from ethanol (260ml) to obtain 17.4gm of pure Sildenafil base.
Purity by HPLC: 99.77% Example 6
Preparation of 5-[2-Ethoxy-5-(4-methylpiperazine-l-yI-sulfonyl)phenyl]-l-methyl- 3-n-propyl-l,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one citrate (Sildenafil Citrate)
Sildenafil base (50 g) was dissolved in acetone (850 ml) at 550C and then slowly added citric acid solution (20 g in 100 ml acetone) over 45 min and maintain the reaction mixture for about 30 min. The reaction mixture was cooled, filtered and dried to get 65 g of Sildenafil citrate.
Purity by HPLC: 99.85%
Chemical synthesis
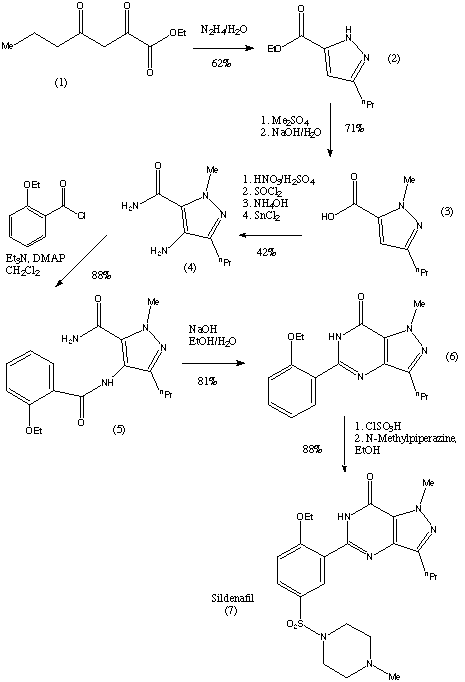
The preparation steps for synthesis of sildenafil are as follows:[45]
- Methylation of 3-propylpyrazole-5-carboxylic acid ethyl ester with hot dimethyl sulfate
- Hydrolysis with aqueous NaOH to free acid
- Nitration with oleum/fuming nitric acid
- Carboxamide formation with refluxing thionyl chloride/NH4OH
- Reduction of nitro group to amino
- Acylation with 2-ethoxybenzoyl chloride
- Cyclization
- Sulfonation to the chlorosulfonyl derivative
- Condensation with 1-methylpiperazine.
SYNTHESIS

...................................................

...........................................................................
SYNTHESIS

......................................................................................

.................................
PRECURSORS
.........................................
SYNTHESIS

Patent issues and expirations
European Union
Pfizer's patent on sildenafil citrate expired in some member countries of the EU, Austria, Denmark, France, Germany, Ireland, Italy, The Netherlands, Spain, Sweden, United Kingdom as well as Switzerland on 21 June 2013.[60][61][62] A UK patent held by Pfizer on the use of PDE5 inhibitors (see below) as treatment of impotence was invalidated in 2000 because of obviousness; this decision was upheld on appeal in 2002.[63][64]
United States
In 1992 Pfizer filed a patent covering the substance sildenafil and its use to treat cardiovascular diseases.[65] This patent was published in 1993 and expired in 2012. In 1994 Pfizer filed a patent covering the use of sildenafil to treat erectile dysfunction.[66] This patent was published in 2002 and will expire in 2019. Teva sued to have the latter patent invalidated, but Pfizer prevailed in an August 2011 federal district court case.[67]
The patent on Revatio (indicated for pulmonary arterial hypertension rather than erectile dysfunction) expired in late 2012. Generic versions of this low-dose form of sildenafil have been available in the U.S. from a number of manufacturers including Greenstone, Mylan and Watson, since early 2013.[68] There is no legal barrier to doctors prescribing this form of sildenafil "off label" for erectile dysfunction, although the dosage typically required for treating ED requires patients to take multiple pills.
Canada
In Canada, Pfizer's patent 2,324,324 for Revatio (sildenafil used to treat pulmonary hypertension) was found invalid by the Federal Court in June 2010, on an application by Ratiopharm Inc.[69][70]
On November 8, 2012 the Supreme Court of Canada ruled that Pfizer's patent 2,163,446 on Viagra was invalid from the beginning because the company did not provide full disclosure in its application. The decision, Teva Canada Ltd. v. Pfizer Canada Inc., pointed to section 27(3)(b) of The Patent Act which requires that disclosure must include sufficient information "to enable any person skilled in the art or science to which it pertains" to produce it. It added further: "As a matter of policy and sound statutory interpretation, patentees cannot be allowed to 'game' the system in this way. This, in my view, is the key issue in this appeal."[71]
Teva Canada launched Novo-Sildenafil, a generic version of Viagra, on the day the Supreme Court of Canada released its decision.[72][73][74] To remain competitive, Pfizer then reduced the price of Viagra in Canada.[75] However, on November 9, 2012, Pfizer filed a motion for a re-hearing of the appeal in the Supreme Court of Canada,[76] on the grounds that the court accidentally exceeded its jurisdiction by voiding the patent.[77] Finally, on April 22, 2013, The Supreme Court of Canada invalidated Pfizer's patent altogether.[78]
India
Manufacture and sale of sildenafil citrate drugs known as "generic viagra" is common in India, where Pfizer's patent claim does not apply. Trade names include Kamagra (Ajanta Pharma), Silagra (Cipla), Edegra (Sun Pharmaceutical), Penegra (Zydus Cadila), and Zenegra (Alkem Laboratories).
China
Manufacture and sale of sildenafil citrate drugs known as "generic viagra" is common in China, where Pfizer's patent claim is not widely enforced.
Other countries
Egypt approved Viagra for sale in 2002, but soon afterwards allowed local companies to produce generic versions of the drug, citing the interests of poor people who would not be able to afford Pfizer's price.[79]
Pfizer's patent on sildenafil citrate expired in Brazil in 2010.[80]
References
- ^ a b Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, Osterloh IH, Gingell C (June 1996). "Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction". Int. J. Impot. Res. 8 (2): 47–52.PMID 8858389.
- ^ Vardi M, Nini A (2007). "Phosphodiesterase inhibitors for erectile dysfunction in patients with diabetes mellitus". In Vardi, Moshe. Cochrane Database Syst Rev (1): CD002187.doi:10.1002/14651858.CD002187.pub3. PMID 17253475.
- ^ Nurnberg HG, Hensley PL, Gelenberg AJ, Fava M, Lauriello J, Paine S (January 2003)."Treatment of antidepressant-associated sexual dysfunction with sildenafil: a randomized controlled trial". JAMA 289 (1): 56–64. doi:10.1001/jama.289.1.56. PMID 12503977.
- ^ Nurnberg HG, Hensley PL, Lauriello J, Parker LM, Keith SJ (August 1999). "Sildenafil for women patients with antidepressant-induced sexual dysfunction". Psychiatr Serv 50 (8): 1076–8. PMID 10445658.
- ^ Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S (2008). "Sildenafil Treatment of Women With Antidepressant-Associated Sexual Dysfunction". JAMA 300 (4): 395–404. doi:10.1001/jama.300.4.395. PMID 18647982.
- ^ "FDA Approves Pfizer's Revatio as Treatment for Pulmonary Arterial Hypertension" (Press release). Pfizer. June 6, 2005. Archived from the original on August 28, 2005.
- ^ Richalet JP, Gratadour P, Robach P, et al. (2005). "Sildenafil inhibits altitude-induced hypoxemia and pulmonary hypertension". Am. J. Respir. Crit. Care Med. 171 (3): 275–81.doi:10.1164/rccm.200406-804OC. PMID 15516532.
- ^ Perimenis P (2005). "Sildenafil for the treatment of altitude-induced hypoxaemia". Expert Opin Pharmacother 6 (5): 835–7. doi:10.1517/14656566.6.5.835. PMID 15934909.
- ^ Fagenholz PJ, Gutman JA, Murray AF, Harris NS (2007). "Treatment of high altitude pulmonary edema at 4240 m in Nepal". High Alt. Med. Biol. 8 (2): 139–46.doi:10.1089/ham.2007.3055. PMID 17584008.
- ^ "Pill Identifier". Drugs.com. Retrieved 2009-02-10. "This site is intended for viewing by the USA audience only. If you are in another country, local laws may not permit access to the medical information contained in this site."
- ^ a b c "Viagra Prescribing Information" (PDF). Pfizer. October 2007. Archived from the original on 14 November 2012. Retrieved 14 November 2012.
- ^ "Viagra and vision". VisionWeb. 29 October 2001. Retrieved 2009-02-10.
- ^ "FDA Updates Labeling for Viagra, Cialis and Levitra for Rare Post-Marketing Reports of Eye Problems". United States Food and Drug Administration. 8 July 2005. Archived from the original on February 23, 2008. Retrieved 2009-02-10.
- ^ Pomeranz HD, Bhavsar AR (March 2005). "Nonarteritic ischemic optic neuropathy developing soon after use of sildenafil (viagra): a report of seven new cases". J Neuroophthalmol 25 (1): 9–13. doi:10.1097/00041327-200503000-00003. PMID 15756125.
- ^ Egan R, Pomeranz H (February 2000). "Sildenafil (Viagra) associated anterior ischemic optic neuropathy". Arch. Ophthalmol. 118 (2): 291–2. PMID 10676804.
- ^ Pomeranz HD, Smith KH, Hart WM, Egan RA (March 2002). "Sildenafil-associated nonarteritic anterior ischemic optic neuropathy". Ophthalmology 109 (3): 584–7. doi:10.1016/S0161-6420(01)00976-9. PMID 11874765.
- ^ Cunningham AV, Smith KH (March 2001). "Anterior ischemic optic neuropathy associated with viagra". J Neuroophthalmol 21 (1): 22–5. doi:10.1097/00041327-200103000-00006.PMID 11315976.
- ^ Boshier A, Pambakian N, Shakir SA (September 2002). "A case of nonarteritic ischemic optic neuropathy (NAION) in a male patient taking sildenafil". Int J Clin Pharmacol Ther 40 (9): 422–3. PMID 12358159.
- ^ Akash R, Hrishikesh D, Amith P, Sabah S (August 2005). "Case report: association of combined nonarteritic anterior ischemic optic neuropathy (NAION) and obstruction of cilioretinal artery with overdose of Viagra". J Ocul Pharmacol Ther 21 (4): 315–7.doi:10.1089/jop.2005.21.315. PMID 16117695.
- ^ "FDA Announces Revisions to Labels for Cialis, Levitra and Viagra". United States Food and Drug Administration. 18 October 2007. Archived from the original on 11 July 2007. Retrieved 10 February 2009.
- ^ "Viagra (sildenafil citrate) tablets". page 29: Pzifer. October 2007. Retrieved 2009-10-25.
- ^ Kloner RA (2005). "Pharmacology and drug interaction effects of the phosphodiesterase 5 inhibitors: focus on alpha-blocker interactions". Am J Cardiol 96 (12B): 42M–46M.doi:10.1016/j.amjcard.2005.07.011. PMID 16387566.
- ^ Cheitlin MD, Hutter AM Jr, Brindis RG, Ganz P, Kaul S, Russell RO Jr, Zusman RM (1999). "ACC/AHA expert consensus document. Use of sildenafil (Viagra) in patients with cardiovascular disease. American College of Cardiology/American Heart Association". Journal of the American College of Cardiology 33 (1): 273–82. doi:10.1016/S0735-1097(98)00656-1.PMID 9935041.
- ^ Peterson K (2001-03-21). "Young men add Viagra to their drug arsenal". USAToday.
- ^ a b c d e Smith KM, Romanelli F (2005). "Recreational use and misuse of phosphodiesterase 5 inhibitors". J Am Pharm Assoc (2003) 45 (1): 63–72; quiz 73–5.doi:10.1331/1544345052843165. PMID 15730119.
- ^ Sildenafil Will Not Affect Libido - Fact!
- ^ a b Mondaini N, Ponchietti R, Muir GH, Montorsi F, Di Loro F, Lombardi G, Rizzo M (June 2003). "Sildenafil does not improve sexual function in men without erectile dysfunction but does reduce the postorgasmic refractory time". Int. J. Impot. Res. 15 (3): 225–8.doi:10.1038/sj.ijir.3901005. PMID 12904810.
- ^ McCambridge J, Mitcheson L, Hunt N, Winstock A (March 2006). "The rise of Viagra among British illicit drug users: 5-year survey data". Drug Alcohol Rev 25 (2): 111–3.doi:10.1080/09595230500537167. PMID 16627299.
- ^ Eloi-Stiven ML, Channaveeraiah N, Christos PJ, Finkel M, Reddy R (November 2007). "Does marijuana use play a role in the recreational use of sildenafil?". J Fam Pract 56 (11): E1–4.PMID 17976333.
- ^ "The 2007 Ig Nobel Prize Winners". Improbable Research. 4 October 2007. Retrieved 2009-02-10.
- ^ Agostino PV, Plano SA, Golombek DA (June 2007). "Sildenafil accelerates reentrainment of circadian rhythms after advancing light schedules". Proc. Natl. Acad. Sci. U.S.A. 104 (23): 9834–9. doi:10.1073/pnas.0703388104. PMC 1887561. PMID 17519328.
- ^ Teri Thompson, Christian Red, Michael O'Keefffe, and Nathaniel Vinton (10 June 2008)."Source: Roger Clemens, host of athletes pop Viagra to help onfield performance". Daily News (Daily News). Retrieved 2009-02-10.
- ^ Busbee J (2012-11-28). "Bears’ Brandon Marshall says some NFL players use Viagra … ON THE FIELD". Yahoo! Sports. Retrieved 2012-11-28.
- ^ Siegel-Itzkovich J (July 1999). "In brief: Viagra makes flowers stand up straight". BMJ 319(7205): 274B. doi:10.1136/bmj.319.7205.274a. PMC 1126921. PMID 10426724.
- ^ "Prolongation of the shelf life of fruits and flowers". Biology Department, University of Hamburg. Retrieved 28 November 2012.
- ^ Oh SS, Zou P, Low MY, Koh HL. Detection of sildenafil analogues in herbal products for erectile dysfunction (2006). "Detection of sildenafil analogues in herbal products for erectile dysfunction". Journal of Toxicology and Environmental Health Part A 69 (21): 1951–1958.doi:10.1080/15287390600751355. PMID 16982533.
- ^ Venhuis BJ, Blok-Tip L, de Kaste D (2008). "Designer drugs in herbal aphrodisiacs".Forensic Science International 177 (2–3): 25–27. doi:10.1016/j.forsciint.2007.11.007.PMID 18178354.
- ^ FDA letter to Libidus distributor
- ^ FDA Warns Consumers About Dangerous Ingredients in "Dietary Supplements" Promoted for Sexual Enhancement
- ^ Hidden Risks of Erectile Dysfunction "Treatments" Sold Online
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 9th edition, Biomedical Publications, Seal Beach, CA, 2011, pp. 1552–1553.http://www.biomedicalpublications.com/dt9.pdf
- ^ Sung, B. J.; Hwang, K.; Jeon, Y.; Lee, J. I.; Heo, Y. S.; Kim, J.; Moon, J.; Yoon, J.; Hyun, Y. L.; Kim, E.; Eum, S.; Park, S. Y.; Lee, J. O.; Lee, T.; Ro, S.; Cho, J. (2003). "Structure of the catalytic domain of human phosphodiesterase 5 with bound drug molecules". Nature 425 (6953): 98–102. doi:10.1038/nature01914. PMID 12955149.
- ^ a b Webb, D.J.; Freestone, S.; Allen, M.J.; Muirhead, G.J. (March 4, 1999). "Sildenafil citrate and blood-pressure-lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist". Am. J. Cardiol. 83 (5A): 21C–28C. doi:10.1016/S0002-9149(99)00044-2. PMID 10078539.
- ^ "Viagra Clinical Pharmacology". RxList.com. 2008. Retrieved 2008-08-20.
- ^ Dunn PJ (2005). "Synthesis of Commercial Phosphodiesterase(V) Inhibitors". Org Process Res Dev 2005 (1): 88–97. doi:10.1021/op040019c.
- ^ "Research". ABM. Abertawe Bro Morgannwg University Health Board. 4 July 2008. Archived from the original on 26 September 2008. Retrieved 6 August 2008. "Our clinicians regularly offer patients the opportunity to take part in trials of new drugs and treatments. Morriston Hospital in Swansea, was the first in the world to trial Viagra!"
- ^ Terrett NK, Bell AS, Brown D, Elllis P (1996). "Sildenafil (Viagra), a potent and selective inhibitor of Type 5 cGMP phosphodiesterase with utility for the treatment of male erectile dysfunction". Bioorg Med Chem Lett 6 (15): 1819–1824. doi:10.1016/0960-894X(96)00323-X.
- ^ Kling J (1998). "From hypertension to angina to Viagra". Mod. Drug Discov. 1: 31–38.ISSN 1532-4486. OCLC 41105083.
- ^ Viagra sales peak at $1,934m in 2008
- ^ Bellis M. "Viagra, the patenting of an aphrodisiac". About.com. Retrieved 2009-02-10.
- ^ Ciment, J (1999). "Missouri fines internet pharmacy". BMJ (British Medical Journal) 319(7221): 1324. doi:10.1136/bmj.319.7221.1324g. PMC 1174637. PMID 10567131.
- ^ Devine, Amy (September 29, 2008). "Chemists plan to sell Viagra on the internet". Daily Record. Retrieved 2012-04-30.
- ^ "Urban Dictionary: Vitamin V". Urban Dictionary. January 16, 2009. Retrieved 2013-12-27.
- ^ Keith A (2000). "The economics of Viagra". Health Aff (Millwood) 19 (2): 147–57.doi:10.1377/hlthaff.19.2.147. PMID 10718028.
- ^ McGuire S (2007-01-01). "Cialis gaining market share worldwide". Medical Marketing & Media. Haymarket Media. Archived from the original on 2009-01-03. Retrieved 2009-02-10.
- ^ Mullin, Rick (June 20, 2005). "Viagra". Chemical & Engineering News 83 (25). Retrieved 2008-08-20.
- ^ Berenson, Alex (December 4, 2005). "Sales of Impotence Drugs Fall, Defying Expectations". The New York Times. Retrieved 2013-12-27.
- ^ "Over-the-counter Viagra piloted". BBC News. BBC News. 11 February 2007. Retrieved 2009-02-10.
- ^ "Pfizer to sell Viagra online, in first for Big Pharma: AP". CBS News. Retrieved 6 May 2013.
- ^ "Actavis Launches Generic Viagra in Europe as Patents Expire". Retrieved 2013-10-25.
- ^ Jim Edwards (October 21, 2009). "What Will Happen When Viagra Goes Generic?". AccessRx.com. Retrieved 2013-12-27.
- ^ "Is Viagra about to lose its pulling power in the UK?". The Guardian. Retrieved 13 June 2013.
- ^ Murray-, Rosie (23 January 2002). "Viagra ruling upsets Pfizer". London: Telegraph Media Group Limited. Archived from the original on 22 August 2009. Retrieved 10 February 2009.
- ^ "Pfizer Loses UK Battle on Viagra Patent". UroToday. Thomson Reuters. 17 June 2002. Archived from the original on 25 June 2007. Retrieved 10 February 2009.
- ^ U.S. Patent 5,250,534
- ^ U.S. Patent 6,469,012
- ^ "Pfizer Wins Viagra Patent Infringement Case Against Teva Pharmaceuticals". Bloomberg. August 15, 2011. Retrieved 2012-04-01.
- ^ "Pfizer's Revatio Goes Generic". Zacks Equity Research. November 15, 2012. Retrieved 2013-10-05.
- ^ "Revation patent ruled invalid for lack of sound prediction and obviousness". Canadian Technology & IP Law. Stikeman Elliott. 2010-06-18. Retrieved 2012-11-14.
- ^ "Pfizer Canada Inc. v. Ratiopharm Inc., 2010 FC 612". CanLII.
- ^ Teva Canada Ltd. v. Pfizer Canada Inc. 2012 SCC 60 at par. 80 (8 November 2012)
- ^ John Spears (2012-11-08). "Supreme Court ruling could lead to cheaper versions of Viagra". The [Toronto] Star. Retrieved 2012-11-14.
- ^ Ken Hanly (2012-11-08). "Canadian Supreme court rules Viagra patent invalid". Digital Journal. Retrieved 2012-11-14.
- ^ "Viagra patent tossed out by Supreme Court: Decision allows generic versions of drug to be produced". CBC News. 2012-11-08. Retrieved 2012-11-14.
- ^ "Pfizer Canada drops Viagra price after generic versions get Supreme Court green light".Financial Post. 2012-11-22. Retrieved 2013-12-27.
- ^ "SCC Case Information, Docket No. 33951". Retrieved 2012-11-14.
- ^ Kirk Makin (2012-11-15). "In rare move, Pfizer asks Supreme Court to reconsider ruling that killed Viagra patent". The Globe and Mail. Retrieved 2012-11-15.
- ^ Gowling Lafleur Henderson LLP, Hélène D'Iorio (2013-04-22). "The Supreme Court of Canada holds Pfizer’s Viagra patent invalid". Lexology. Retrieved 2013-12-27.
- ^ Allam, Abeer (October 4, 2002). "Seeking Investment, Egypt Tries Patent Laws". New York Times. Retrieved 2013-12-27.
- ^ Viagra patent expires in June, says Brazilian court
External links
Official Viagra Website- Official Viagra UK Website
- Official Revatio Website
- prescribing information for Viagra and prescribing information for Revatio from Pfizer
- FDA Information
- MedlinePLUS information, including side effects
- U.S. National Library of Medicine: Drug Information Portal – Sildenafil
- Viagra bound to proteins in the PDB
- Viagra at The Periodic Table of Videos (University of Nottingham)