A condition called high cholesterol slowly creeps on you and you are unable to see its ill-effects until it becomes unbearable with spike to several complications such as diabetes, heart diseases and much more.
Other than just to pop up pills to keep the level of bad cholesterol or LDL under check, it is always a good choice to go for 5 must to have foods to keep the levels of high cholesterol under control. Thus, let’s go straight and see them. |
Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, helping millions with websites, 9 million hits on google, 2.5 lakh connections worldwide, P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
Showing posts with label POLYMORPHISM. Show all posts
Showing posts with label POLYMORPHISM. Show all posts
Friday, 26 July 2013
5 Must To Have Foods To Keep The Levels Of High Cholesterol Under Control
Why Vertex Earnings Prospects Are So Bright
Why Vertex Earnings Prospects Are So Bright
Motley Fool
With few drugs targeting cystic fibrosis and almost half of patients suffering from the particular mutation that the study targeted, Vertex could well have identified a blockbuster in the space. Moreover, with an ongoing phase 3 trial of another ...
http://www.fool.com/investing/general/2013/07/25/why-vertex-earnings-prospects-are-so-bright.aspx
Vertex Pharmaceuticals has a couple of approved drugs, including its Kalydeco cystic fibrosis drug and its Incivek treatment for hepatitis C. But it also has some promising prospects in the development stage. With the company having reported some positive study results during the quarter, Vertex saw its shares soar as more investors got aboard the biotech's bandwagon. Let's take an early look at what's been happening with Vertex Pharmaceuticals over the past quarter and what we're likely to see in its quarterly report
Motley Fool
With few drugs targeting cystic fibrosis and almost half of patients suffering from the particular mutation that the study targeted, Vertex could well have identified a blockbuster in the space. Moreover, with an ongoing phase 3 trial of another ...
http://www.fool.com/investing/general/2013/07/25/why-vertex-earnings-prospects-are-so-bright.aspx
Vertex Pharmaceuticals has a couple of approved drugs, including its Kalydeco cystic fibrosis drug and its Incivek treatment for hepatitis C. But it also has some promising prospects in the development stage. With the company having reported some positive study results during the quarter, Vertex saw its shares soar as more investors got aboard the biotech's bandwagon. Let's take an early look at what's been happening with Vertex Pharmaceuticals over the past quarter and what we're likely to see in its quarterly report
Monday, 22 July 2013
Cancer drug tested in pet dogs is now bound for human trials

PAC 1
Thanks to a new $2 million investment, a drug that spurs cancer cells to self-destruct while sparing healthy cells is on the road to human clinical trials. The compound, known as PAC-1, has so far proven safe and has promising anti-cancer effects in cell culture, in mouse models of cancer and in pet dogs with spontaneously occurring lymphomas and osteosarcomas.
If PAC-1 (pack one) makes it through the U.S. Food and Drug Administration’s Investigational New Drug review, the first human (Phase I) clinical trial of the drug will begin in mid-2014. The investor, who wishes to remain anonymous, has an option to invest another $2 million to take the drug into human trials. The clinical work will be conducted at the University of Illinois Cancer Center in Chicago.
http://news.illinois.edu/news/13/0717cancer_drug_PaulHergenrother_TimFan.html

Photo by
L. Brian Stauffer
University of Illinois chemistry professor Paul Hergenrother, left, and veterinary clinical medicine professor Tim Fan led a study of an anti-cancer compound in pet dogs that is now headed for human clinical trials.
PAC-1 (first procaspase activating compound) is a synthesized chemical compound that selectively induces apoptosis, or cell suicide, in cancerous cells. PAC-1 has shown good results in mouse models and is being further evaluated for use in humans. In 2010 a published study showed PAC-1 to be safe to research dogs, and a second study published later that same year reported that a PAC-1 derivative (called S-PAC-1) was well tolerated in a small Phase I Clinical Trial of pet dogs with lymphoma. Even at low doses of S-PAC-1, tumors regressed in 1/6 dogs, and the disease was stabilized (no additional tumor growth) in 3/6 dogs
L. Brian Stauffer
University of Illinois chemistry professor Paul Hergenrother, left, and veterinary clinical medicine professor Tim Fan led a study of an anti-cancer compound in pet dogs that is now headed for human clinical trials.
PAC-1 (first procaspase activating compound) is a synthesized chemical compound that selectively induces apoptosis, or cell suicide, in cancerous cells. PAC-1 has shown good results in mouse models and is being further evaluated for use in humans. In 2010 a published study showed PAC-1 to be safe to research dogs, and a second study published later that same year reported that a PAC-1 derivative (called S-PAC-1) was well tolerated in a small Phase I Clinical Trial of pet dogs with lymphoma. Even at low doses of S-PAC-1, tumors regressed in 1/6 dogs, and the disease was stabilized (no additional tumor growth) in 3/6 dogs
Saturday, 13 July 2013
Friday, 12 July 2013
Vical's Allovectin Phase III Trial Results: Consider The Possibilities
|
Cohen: We saw that happen last year when JNJ prematurely unblinded the pre- chemo Phase III study for the prostate cancer drug Zytiga. The trial achieved ... 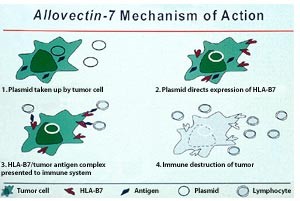 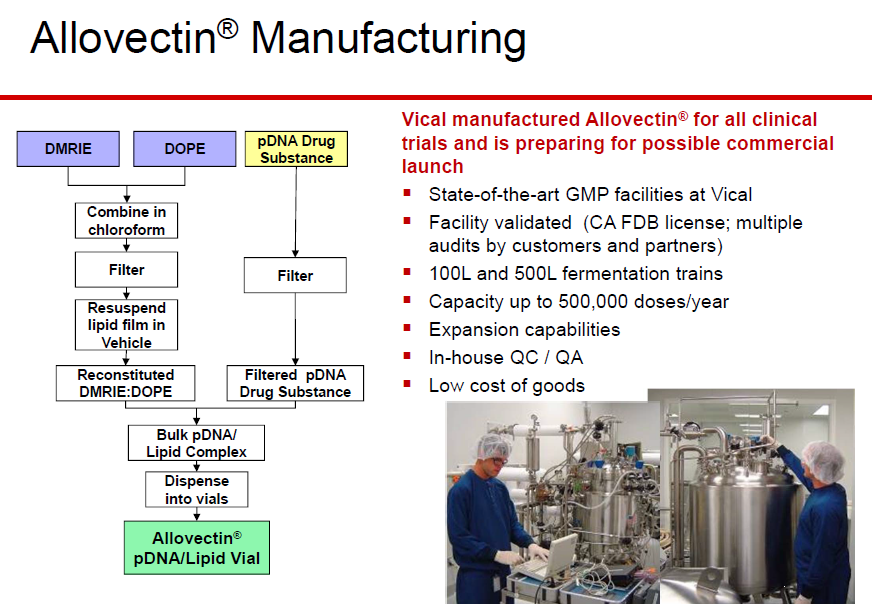 In 1999, FDA granted Allovectin-7 orphan drug designation for the treatment of invasive and metastatic melanoma.
|
Monday, 8 July 2013
University of East Anglia scientists make major advance important for cancer research
 Above: EB2 (green) expressed in stem cell region of gut with microtubules in red (credit: Deborah Goldspink)
Above: EB2 (green) expressed in stem cell region of gut with microtubules in red (credit: Deborah Goldspink)
University of East Anglia scientists make major advance important for cancer research

Thursday, 4 July 2013
Chocolate as medicine: a quest over the centuries
The rehabilitation of chocolate has occurred only in recent times. The pages of scientific magazines have been positively recaptured and chocolate’s reputation is being restored to the value that Carl Linnaeus credited to this food, when he named the generous plant Theobroma cacao, the food of the gods
read all at
http://www.rsc.org/chemistryworld/2013/07/chocolate-medicine-wilson-hurst

Monday, 1 July 2013
Verona Pharma Plc Peer-Reviewed Paper Suggests RPL554 With Glycopyrrolate, Or Other Muscarinic Receptor Antagonists, Produces Synergistic Bronchodilation


RPL554
Verona Pharma Plc ("Verona Pharma" Or The "Company") Peer-Reviewed ...
Wall Street Journal
Verona Pharma is developing first-in-class drugs to treat respiratory disease, such as COPD, asthma and chronic, severe cough. The Company has three drug programmes, two of which are in Phase II. The lead programme, RPL554, is an innovative dual ...
read all at
http://online.wsj.com/article/PR-CO-20130701-900428.html?mod=googlenews_wsj
RPL-554 (LS-193,855) is a drug which acts as a long-acting inhibitor of the phosphodiesterase enzymes PDE-3 and PDE-4, producing both bronchodilator and antiinflammatory effects.[1] It is being developed by Verona Pharma as a potential treatment for asthma and hay fever, and is currently in clinical trials.[2][3]
- Boswell-Smith V, Spina D, Oxford AW, Comer MB, Seeds EA, Page CP. The Pharmacology of Two Novel Long-Acting Phosphodiesterase 3/4 Inhibitors, RPL554 (9,10-Dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl) -3,4,6,7-tetrahydro-2H-pyrimido(6,1-a)isoquinolin-4-one) and RPL565 (6,7-Dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido(6,1-a)isoquinolin-4-one). Journal of Pharmacology and Experimental Therapeutics 2006; 318(2):840-848.
- Verona Pharma Plc - Lead Drug RPL554
- Asthma and hay fever drug tested. BBC News, Wednesday 10 September 2008
Labels:
anthony crasto,
catalyst,
DRUGS,
hepatitis drug,
herbs,
medicinal chemistry,
New Drugs,
ORGANIC SYNTHESIS,
PATENTS,
POLYMORPHISM,
process,
RPL554,
verona pharma,
world drug tracker
Friday, 28 June 2013
WORLD DRUG TRACKER | join LinkedIn Group
WORLD DRUG TRACKER | LinkedIn
Jun 7, 2013 – To track information on drugs on worldwide basis, all aspects covered, A group by DR ANTHONY MELVIN CRASTO Ph.D.
Alexion’s Soliris® (eculizumab) Receives Orphan Drug Designation for the Treatment of Neuromyelitis Optica (NMO)

Structure of eculizumab. Eculizumab was engineered
to reduce immunogenicity and eliminate effector function. Human IgG2 and IgG4
heavy-chain sequences were combined to form a hybrid constant region that is
unable to bind Fc receptors or to activate the complement cascade. Eculizumab
exhibits high affinity for human C5, effectively blocking its cleavage and
downstream proinflammatory and cell lytic properties. Reprinted from Rother et
al with permission.
Alexion's Soliris® (eculizumab) Receives Orphan Drug Designation for the ...
Fort Mills Times
In a Phase 2 study presented at the 2012 annual meeting of the American Neurological Association (ANA), Soliris treatment was associated with a significant reduction in the frequency of relapses (recurring attacks) in patients with severe, relapsing ...
http://www.fortmilltimes.com/2013/06/27/2789656/alexions-soliris-eculizumab-receives.html
Eculizumab (INN and USAN; trade name Soliris) is a humanized monoclonal antibody that is a first-in-class terminal complement inhibitor and the first therapy approved for the treatment of paroxysmal nocturnal hemoglobinuria (PNH), a rare, progressive, and sometimes life-threatening disease characterized by excessive destruction of red blood cells (hemolysis). It costs £400,000 (US$600,000) per year per patient
Eculizumab also is the first agent approved for the treatment of atypical hemolytic uremic syndrome (aHUS), an ultra-rare genetic disease that causes abnormal blood clots to form in small blood vessels throughout the body, leading to kidney failure, damage to other vital organs and premature death.
In clinical trials in patients with PNH, eculizumab was associated with reductions in chronic hemolysis, thromboembolic events, and transfusion requirements, as well as improvements in PNH symptoms, quality of life, and survival.Clinical trials in patients with aHUS demonstrated inhibition of thrombotic microangiopathy (TMA),the formation of blood clots in small blood vessels throughout the body, including normalization of platelets and lactate dehydrogenase (LDH), as well as maintenance or improvement in renal function.
Eculizumab was discovered and developed by Alexion Pharmaceuticals and is manufactured by Alexion. It was approved by the United States Food and Drug Administration (FDA) on March 16, 2007 for the treatment of PNH, and on September 23, 2011 for the treatment of aHUS. It was approved by the European Medicines Agency for the treatment of PNH on June 20, 2007, and on November 24, 2011 for the treatment of aHUS. Eculizumab is currently being investigated as a potential treatment for other severe, ultra-rare disorders.
Labels:
anthony crasto,
ayurveda,
catalyst,
DRUGS,
eculizumab,
herbs,
medicinal chemistry,
New Drugs,
organic chemistry,
ORGANIC SYNTHESIS,
PATENTS,
POLYMORPHISM,
process,
world drug tracker
Celgene buys MophoSys for myeloma antibody development
Celgene buys MophoSys for myeloma antibody development
German biopharmaceutical company MorphoSys will jointly develop an antibody for the treatment of multiple myeloma (MM) and leukaemia with Celgene Corporation.
http://www.pharmaceutical-technology.com/news/newscelegene-buys-mophosys-for-myeloma-antibody-development?WT.mc_id=DN_News

Tuesday, 25 June 2013
How many modes of action should an antibiotic have?

Structures of resistance-breaking derivatives of established antibiotic classes. Selected compounds are depicted that were recently launched or are currently in development. Ceftobiprole has increased affinity for PBP2a, a member of the target family of penicillin-binding proteins not affected by marketed β-lactams. Tigecycline, iclaprim, telithromycin, and telavancin make contacts to additional binding sites on their established targets or address additional targets. Structural elements responsible for the novel target interactions are marked bold. MCB-3681, TD-1792, and CBR-2092 are hybrid molecules, in which two pharmacophors from different antibiotic classes are attached by linkers. Linkers are marked bold
All antibiotics that have been successfully employed for decades as monotherapeutics in the treatment of bacterial infections rely on mechanisms of bacterial growth inhibition which are by far more complex than inhibition of a single enzyme. Such successful antibiotics have in common that they address several targets in parallel and/or that their targets are encoded by multiple genes. Such multiplicity of targets and of target genes has the advantage that the emergence of spontaneous target-related resistance is a comparatively slow process. Recently registered antibiotics and novel antibiotics in development are discussed in the light of this promising concept of antibacterial polypharmacology.
How many modes of action should an antibiotic have?
- AiCuris GmbH & Co.KG, Friedrich-Ebert Strasse 475, Building 302, D-42117 Wuppertal, Germany
- Available online 30 July 2008
http://www.sciencedirect.com/science/article/pii/S1471489208000799

Monday, 24 June 2013
Preventing Dangerous Liaisons
 A new peptidomimetic inhibits prostate cancer’s growth by
preventing the androgen receptor from binding to its cofactor
A new peptidomimetic inhibits prostate cancer’s growth by
preventing the androgen receptor from binding to its cofactorRead more
http://www.chemistryviews.org/details/news/4884471/Preventing_Dangerous_Liaisons.html
Friday, 21 June 2013
.Artificial sweetener a potential treatment for Parkinson’s disease.
Mannitol, a sugar alcohol produced by fungi, bacteria,
and algae, is a common component of sugar-free gum and candy. The sweetener is
also used in the medical field — it’s approved by the FDA as a diuretic to flush
out excess fluids and used during surgery as a substance that opens the
blood/brain barrier to ease the passage of other drugs.
Now Profs. Ehud Gazit and Daniel Segal of Tel Aviv University‘s Department of Molecular Microbiology
and Biotechnology and the Sagol School of Neuroscience, along with their
colleague Dr. Ronit Shaltiel-Karyo and PhD candidate Moran Frenkel-Pinter, have
found that mannitol also prevents clumps of the protein α-synuclein from forming
in the brain — a process that is characteristic of Parkinson’s disease.Read more at
Tuesday, 18 June 2013
Monday, 17 June 2013
Ibrutinib Phase 2 Data: Analyses Show Efficacy with Ibrutinib Monotherapy in Patients with Relapsed or Refractory Mantle Cell or Diffuse Large B-cell Lymphoma
ibrutinib
June 16, 2013
Janssen Research & Development, LLC (Janssen), today announced the results of two separate Phase 2 studies suggesting that ibrutinib, an investigational oral Bruton's tyrosine kinase (BTK) inhibitor, shows efficacy when used as a monotherapy in patients with relapsed/refractory mantle cell lymphoma (MCL) or diffuse large B-cell lymphoma (DLBCL). The studies were presented today at the European Hematology Association (EHA) 18th Annual Congress in Stockholm, Sweden. Ibrutinib is being jointly developed by Janssen and Pharmacyclics, Inc.
Ibrutinib (USAN), also known as PCI-32765, is an experimental drug candidate for the treatment of various types of cancer. It is an orally-administered, selective and covalent inhibitor of the enzyme Bruton tyrosine kinase (Btk).
Ibrutinib is currently under development by Pharmacyclics, Inc and Johnson & Johnson's Janssen Pharmaceutical division for B-cell malignancies including chronic lymphocytic leukemia, mantle cell lymphoma, diffuse large B-cell lymphoma, and multiple myeloma.
Ibrutinib was first designed and synthesized at Celera Genomics by Zhengying Pan, who along with a team of chemists and biologists reported in 2007 a structure-based approach for creating a series of small molecules that inactivate BTK through covalent binding to cysteine-481 near the ATP binding domain of BTK[2].
These small molecules irreversibly inhibited BTK by using a Michael acceptor for binding to the target cysteine.
In April 2006, Pharmacyclics acquired Celera’s small molecule BTK inhibitor discovery program, which included a compound, PCI-32765 (known as compound 13 in the Pan et al paper) that was subsequently chosen for further preclinical development based on the discovery of anti-lymphoma properties in vivo .
Since 2006, Pharmacyclics’ scientists have advanced the molecule into clinical trials and identified specific clinical indications for the drug. It also has potential effects against autoimmune arthritis.
Sunday, 16 June 2013
Pharma-Execs-2012-Pipeline-Report
Just days before this article went to press, FDA approved the first of a new kind of oral enzyme treatment that mediates cellular response, Incyte/Novartis' Jakafi, for a rare bone marrow disease called myelofibrosis. The next JAK inhibitor, Pfizer's toficitinib, could hit the market late next year, meaning a lot of rheumatoid arthritis patients will never again have to sit in a hospital for a couple of hours to get an anti-TNF infusion. Many innovative drugs, long out of the gate, are closing in on the finish line; science is back, and a better understanding of the way genomics shapes disease is bringing about better outcomes, and sometimes faster approvals.
read all at
http://www.pharmexec.com/pharmexec/Deals/Pharm-Execs-2012-Pipeline-Report/ArticleStandard/Article/detail/752361?contextCategoryId=48159
Friday, 14 June 2013
The Design, Development and Scale-Up of Safe Chemical Processes and Operations
2 - 4 October 2013 •
Chilworth Global, Princeton, NJ, USA
Dr Vahid Ebadat, Chilworth Technology Inc.
Dr. Swati Umbrajkar
Dr Will Watson, Scientific Update
Employers are bound by Health & Safety legislation to ensure the safety of their employees and those outside their employment who might be affected by their activities. Chemical manufacturers must therefore be aware of all potential dangers in their processes and take steps to eliminate them. The best approach is to design safety into the process from the start.
This seminar is designed to enhance the awareness of chemists and engineers regarding hazard issues. Utilizing the expertise of the chemists and chemical engineers at Chilworth Global and Scientific Update, it will consider hazard control of new chemical processes throughout their development cycle: from early development through to full-scale production. Hazards can often be eliminated by appropriate choice of reagent or synthetic route at the R&D stage. Where this is not possible, techniques exist to quantify the hazards so that robust engineering solutions can he applied in production.
Who Should Attend?
- R&D and Process Development Chemists, Chemical Engineers, Managers and anyone whose responsibilities include safety or risk assessment of chemical processes or building safety into chemical process scale-up.
Visit SCIENTIFIC UPDATE website for complete course information.
Subscribe to:
Comments (Atom)





