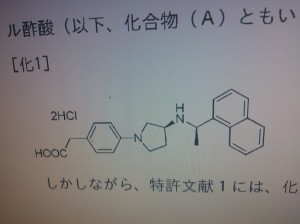
KHK 7580 .....example no 3.001 R1-X- group\



HCl salt
MS · APCI: 361 [M+H] +

in EP1757582
4-(3S-(1R-(1-naphthyl)ethylamino)pyrrolidin-1- yl)phenylacetic acid
4-[(3S)-3-[[(1R)-1-(1-naphthalenyl)ethyl]amino]-1-pyrrolidinyl]-Benzeneacetic acid,
cas will be updated
BASE ....870964-67-3
DI HCL SALT .......870856-31-8
MF C24 H26 N2 O2 BASE
MW 374.48 BASE
KHK-7580
KHK-7580; MT-4580
Mitsubishi Tanabe Pharma Corp... innovator
Kyowa Hakko Kirin Co Ltd.. licencee
4-(3S-(1R-(1-naphthyl)ethylamino)pyrrolidin-1-yl)phenylacetic acid,
useful as calcium-sensitive receptor (CaSR) agonists for treating hyperparathyroidism. a CaSR agonist, being developed by Kyowa Hakko Kirin, under license from Mitsubishi Tanabe, for treating secondary hyperparathyroidism (phase 2 clinical, as of March 2015).
WILL BE UPDATED
WO2005115975,/EP1757582
http://www.google.co.in/patents/EP1757582A1?cl=en
Example no 3.001 R1-X- group



HCl salt
MS · APCI: 361 [M+H] +

WO 2015034031A1
http://worldwide.espacenet.com/publicationDetails/biblio?DB=worldwide.espacenet.com&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20150312&CC=WO&NR=2015034031A1&KC=A1
Mitsubishi Tanabe Pharma Corporation
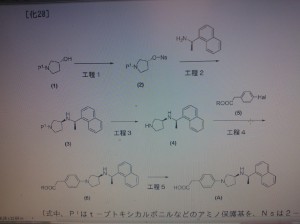
The present invention provides a novel crystal form of an arylalkylamine
compound. Specifically, a novel crystal form of
4-(3S-(1R-(1-naphthyl)ethylamino)pyrrolidin-1- yl)phenylacetic acid has
excellent stability, and is therefore useful as an active ingredient for
a medicine. The present invention also provides an industrially
advantageous method for producing an arylalkylamine compound.
WO 2015034031A1
http://worldwide.espacenet.com/publicationDetails/biblio?DB=worldwide.espacenet.com&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20150312&CC=WO&NR=2015034031A1&KC=A1
Mitsubishi Tanabe Pharma Corporation
The present invention provides a novel crystal form of an arylalkylamine compound. Specifically, a novel crystal form of 4-(3S-(1R-(1-naphthyl)ethylamino)pyrrolidin-1- yl)phenylacetic acid has excellent stability, and is therefore useful as an active ingredient for a medicine. The present invention also provides an industrially advantageous method for producing an arylalkylamine compound.
see all at http://drugpatentsint.blogspot.in/2015/03/wo-2015034031.html
see all at http://drugpatentsint.blogspot.in/2015/03/wo-2015034031.html
see all at http://drugpatentsint.blogspot.in/2015/03/wo-2015034031.html
see all at http://drugpatentsint.blogspot.in/2015/03/wo-2015034031.html
see all at http://drugpatentsint.blogspot.in/2015/03/wo-2015034031.html
do not miss out on above click
http://www.kyowa-kirin.com/research_and_development/pipeline/



compound. Specifically, a novel crystal form of
4-(3S-(1R-(1-naphthyl)ethylamino)pyrrolidin-1- yl)phenylacetic acid has
excellent stability, and is therefore useful as an active ingredient for
a medicine. The present invention also provides an industrially
advantageous method for producing an arylalkylamine compound.

WO 2015034031A1
http://worldwide.espacenet.com/publicationDetails/biblio?DB=worldwide.espacenet.com&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20150312&CC=WO&NR=2015034031A1&KC=A1
Mitsubishi Tanabe Pharma Corporation
The present invention provides a novel crystal form of an arylalkylamine compound. Specifically, a novel crystal form of 4-(3S-(1R-(1-naphthyl)ethylamino)pyrrolidin-1- yl)phenylacetic acid has excellent stability, and is therefore useful as an active ingredient for a medicine. The present invention also provides an industrially advantageous method for producing an arylalkylamine compound.
see all at http://drugpatentsint.blogspot.in/2015/03/wo-2015034031.html
see all at http://drugpatentsint.blogspot.in/2015/03/wo-2015034031.html
see all at http://drugpatentsint.blogspot.in/2015/03/wo-2015034031.html
see all at http://drugpatentsint.blogspot.in/2015/03/wo-2015034031.html
see all at http://drugpatentsint.blogspot.in/2015/03/wo-2015034031.html
do not miss out on above click
http://www.kyowa-kirin.com/research_and_development/pipeline/
KHK7580 -Secondary Hyperparathyroidism
JP
This randomized, placebo-controlled, double-blind, parallel-group, multi-center study is designed to evaluate efficacy and safety in cohorts comprising KHK7580, its placebo and cinacalcet and initial dose of KHK7580 for secondary hyperparathyroidism patients receiving hemodialysis.
KHK7580 is a small molecular compound produced by Mitsubishi Tanabe Pharma Corporation (President & Representative Director, CEO: Masayuki Mitsuka, "Mitsubishi Tanabe Pharma"). Kyowa Hakko Kirin signed a license agreement of KHK7580 with Mitsubishi Tanabe Pharma for the rights to cooperative research, develop, market and manufacture the product in Japan and some part of Asia on March 2008.
The Kyowa Hakko Kirin Group is contributing to the health and prosperity of the world's people by pursuing advances in life sciences and technology and creating new value.
Outline of this study
Media Contact:
+81-3-3282-1903
or
Investors:
+81-3-3282-0009
http://www.medkoo.com/products/6729
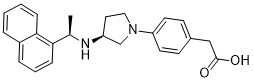
Name: Evocalcet
CAS#: 870964-67-3
Chemical Formula: C24H26N2O2
Exact Mass: 374.19943
Evocalcet is a calcium-sensing receptor agonist. The calcium-sensing receptor (CaSR) is a Class C G-protein coupled receptor which senses extracellular levels of calcium ion. The calcium-sensing receptor controls calcium homeostasis by regulating the release of parathyroid hormone (PTH). CaSR is expressed in all of the organs of the digestive system. CaSR plays a key role in gastrointestinal physiological function and in the occurrence of digestive disease. High dietary Ca2+ may stimulate CaSR activation and could both inhibit tumor development and increase the chemotherapeutic sensitivity of cancer cells in colon cancer tissues. (Last update: 12/15/2015).
Synonym: MT-4580; MT 4580; MT4580; KHK-7580; KHK7580; KHK 7580; Evocalcet
IUPAC/Chemical Name: 2-(4-((S)-3-(((R)-1-(naphthalen-1-yl)ethyl)amino)pyrrolidin-1-yl)phenyl)acetic acid
2
https://tripod.nih.gov/ginas/app/substance/f580b9fd

http://www.drugspider.com/drug/evocalcet
///////////////
SMILES Code: O=C(O)CC1=CC=C(N2C[C@@H](N[C@@H](C3=C4C=CC=CC4=CC=C3)C)CC2)C=C1
| Company | Mitsubishi Tanabe Pharma Corp. |
| Description | Calcium receptor agonist |
| Molecular Target | |
| Mechanism of Action | Calcium-sensing receptor (CaSR) agonist |
| Therapeutic Modality | Small molecule |
| Latest Stage of Development | Phase II |
| Standard Indication | Thyroid disease |
| Indication Details | Treat hyperparathyroidism in patients receiving hemodialysis; Treat secondary hyperparathyroidism (SHPT) |
| Regulatory Designation | |
| Partner |
August 29, 2014
Kyowa Hakko Kirin Announces Commencement of Phase 2b Clinical Study of KHK7580 in Patients with Secondary Hyperparathyroidism in Japan
Tokyo, Japan, August 29, 2014 --- Kyowa Hakko Kirin Co., Ltd. (Tokyo: 4151, President and CEO: Nobuo Hanai, "Kyowa Hakko Kirin") today announced the initiation of a phase 2b clinical study evaluating KHK7580 for secondary hyperparathyroidism patients receiving hemodialysis in Japan.This randomized, placebo-controlled, double-blind, parallel-group, multi-center study is designed to evaluate efficacy and safety in cohorts comprising KHK7580, its placebo and cinacalcet and initial dose of KHK7580 for secondary hyperparathyroidism patients receiving hemodialysis.
KHK7580 is a small molecular compound produced by Mitsubishi Tanabe Pharma Corporation (President & Representative Director, CEO: Masayuki Mitsuka, "Mitsubishi Tanabe Pharma"). Kyowa Hakko Kirin signed a license agreement of KHK7580 with Mitsubishi Tanabe Pharma for the rights to cooperative research, develop, market and manufacture the product in Japan and some part of Asia on March 2008.
The Kyowa Hakko Kirin Group is contributing to the health and prosperity of the world's people by pursuing advances in life sciences and technology and creating new value.
Outline of this study
| ClinicalTrials.gov Identifier | |
|---|---|
| Target Population | Secondary hyperparathyroidism patients receiving hemodialysis |
| Trial Design | Randomized, placebo-controlled, double-blind (included open arm of cinacalcet), parallel-group, multi-center study |
| Administration Group | KHK7580, Placebo, cinacalcet |
| Target Number of Subjects | 150 |
| Primary Objective | Efficacy |
| Trial Location | Japan |
| Trial Duration | Jul. 2014 to Jun. 2015 |
Contact:
Kyowa Hakko KirinMedia Contact:
+81-3-3282-1903
or
Investors:
+81-3-3282-0009
Update on march 2016
New comment waiting approval on New Drug Approvals |
|
http://www.medkoo.com/products/6729

Name: Evocalcet
CAS#: 870964-67-3
Chemical Formula: C24H26N2O2
Exact Mass: 374.19943
Evocalcet is a calcium-sensing receptor agonist. The calcium-sensing receptor (CaSR) is a Class C G-protein coupled receptor which senses extracellular levels of calcium ion. The calcium-sensing receptor controls calcium homeostasis by regulating the release of parathyroid hormone (PTH). CaSR is expressed in all of the organs of the digestive system. CaSR plays a key role in gastrointestinal physiological function and in the occurrence of digestive disease. High dietary Ca2+ may stimulate CaSR activation and could both inhibit tumor development and increase the chemotherapeutic sensitivity of cancer cells in colon cancer tissues. (Last update: 12/15/2015).
Synonym: MT-4580; MT 4580; MT4580; KHK-7580; KHK7580; KHK 7580; Evocalcet
IUPAC/Chemical Name: 2-(4-((S)-3-(((R)-1-(naphthalen-1-yl)ethyl)amino)pyrrolidin-1-yl)phenyl)acetic acid
2
https://tripod.nih.gov/ginas/app/substance/f580b9fd

http://www.drugspider.com/drug/evocalcet
| INN name |
Evocalcet
| ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lab Code(s) |
MT-4580
KHK-7580
| ||||||||||||||||||||||||||||||||||||||||
| Chemical name |
{4-[(3S)-3-{[(1R)-1-(Naphthalen-1-yl)ethyl]amino}pyrrolidin-1-yl]phenyl}acetic acid
| ||||||||||||||||||||||||||||||||||||||||
| Chemical structure |  | ||||||||||||||||||||||||||||||||||||||||
| Molecular formula |
C24H26N2O2
| ||||||||||||||||||||||||||||||||||||||||
| SMILES |
O=C(O)CC1=CC=C(N2C[C@@H](N[C@@H](C3=C4C=CC=CC4=CC=C3)C)CC2)C=C1
| ||||||||||||||||||||||||||||||||||||||||
| CAS registry number |
870964-67-3
| ||||||||||||||||||||||||||||||||||||||||
| Orphan Drug Status |
No
| ||||||||||||||||||||||||||||||||||||||||
| On Fast track |
No
| ||||||||||||||||||||||||||||||||||||||||
| New Molecular Entity |
Yes
| ||||||||||||||||||||||||||||||||||||||||
| Originator | |||||||||||||||||||||||||||||||||||||||||
| Developer(s) | |||||||||||||||||||||||||||||||||||||||||
| Class | |||||||||||||||||||||||||||||||||||||||||
| Mechanism of action | |||||||||||||||||||||||||||||||||||||||||
| WHO ATC code(s) | |||||||||||||||||||||||||||||||||||||||||
| EPhMRA code(s) | |||||||||||||||||||||||||||||||||||||||||
| Clinical trial(s) |
| ||||||||||||||||||||||||||||||||||||||||
| Updated on |
11 Oct 2015
|
SMILES Code: O=C(O)CC1=CC=C(N2C[C@@H](N[C@@H](C3=C4C=CC=CC4=CC=C3)C)CC2)C=C1