Uses
FDA-approved use

Femara 2.5 mg oral tablet
Letrozole is approved by the
United States Food and Drug Administration (FDA) for the treatment of local or metastatic breast cancer that is hormone receptor positive or has an unknown receptor status in postmenopausal women.
[2]
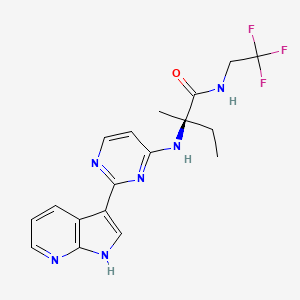
4-[alpha (4-cyanophenyl)-l-(l,2,4-triazoly)-methyl]- benzonitrile
Off-label uses
Letrozole has been used for
ovarian stimulation by fertility doctors since 2001 because it has fewer side-effects than
clomiphene (
Clomid) and less chance of multiple gestation. A Canadian study presented at the
American Society of Reproductive Medicine 2005 Conference suggests that letrozole may increase the risk of birth defects. A more detailed ovulation induction follow-up study found that letrozole, compared with a control group of clomiphene, had significantly lower congenital malformations and chromosomal abnormalities at an overall rate of 2.4% (1.2% major malformations) compared with clomiphene 4.8% (3.0% major malformations).
[3] Despite this, India banned the usage of letrozole in 2011, citing potential risks to infants.
[4] In 2012, an Indian parliamentary committee said that the drug controller office colluded with letrozole's makers to approve the drug for infertility in India and also stated that letrozole's use for infertility was illegal worldwide;
[5] however, such
off-label uses are legal in many countries such as the US and UK.
[6][7]
The anti-estrogen action of letrozole has been shown to be useful in pretreatment for termination of pregnancy, in combination with
misoprostol. It can be used in place of
mifepristone, which is expensive and unavailable in many countries.
[8]
Letrozole is sometimes used as a treatment for
gynecomastia, although it is probably most effective at this if caught in an early stage (such as in users of
anabolic steroids).
[9][10]
Some studies have shown that letrozole can be used to promote spermatogenesis in male patients suffering from nonobstructive
azoospermia.
[11]
Letrozole has also been shown to delay the fusing of the
growth plates in mice.
[12] When used in combination with growth hormone, letrozole has been shown effective in one adolescent boy with a short stature.
[13]
Mechanism of action
Estrogens are produced by the conversion of
androgens through the activity of the
aromatase enzyme. Estrogens then bind to an estrogen receptor, which causes cells to divide.
Letrozole prevents the aromatase from producing estrogens by competitive, reversible binding to the heme of its
cytochrome P450 unit. The action is specific, and letrozole does not reduce production of mineralo- or corticosteroids.
Contraindications
Letrozole is contraindicated in women having a pre-
menopausal hormonal status, during pregnancy and lactation.
[15]
Adverse effects
Generally, side effects include signs and symptoms of
hypoestrogenism. There is concern that long term use may lead to
osteoporosis,
[2] which is in certain patient populations such as post-menopausal women or osteoporotics,
bisphosphonates may also be prescribed.
Interactions
Comparison with tamoxifen
Tamoxifen is also used to treat hormonally-responsive breast cancer, but it does so by interfering with the estrogen receptor. However, letrozole is effective only in post-menopausal women, in whom estrogen is produced predominantly in peripheral tissues (i.e. in adipose tissue, like that of the breast) and a number of sites in the brain.
[16] In pre-menopausal women, the main source of estrogen is from the ovaries not the peripheral tissues, and letrozole is ineffective.
In the BIG 1–98 Study, of post-menopausal women with hormonally-responsive breast cancer, letrozole reduced the recurrence of cancer, but did not change survival rate, compared to tamoxifen.
[17][18]
Synthesis
Letrozole can by synthesized from 4-cyanobenzyl bromide,
triazole, and 4-fluorobenzonitrile:
[19] 
...............................................
Letrozole, chemically known as 4-[alpha (4-cyanophenyl)-1-(1,2,4-triazoly)-methyl]-benzonitrile, and represented by formula (I),
is a therapeutically and commercially important non-steroidal aromatase inhibitor, which is widely used for adjuvant treatment of hormonally responsible breast cancer in postmenopausal women. Estrogens are produced by the conversion of androgen through the activity of aromatase enzyme, the suppression of estrogen biosynthesis in peripheral tissues and in the cancer tissue itself can therefore be achieved by specifically inhibiting the aromatase enzyme.
[0003]
1. Bowman et al. were the first to disclose Letrozole in
US 4,978,672 , and
US 5,352,795 and reported two methods for synthesis of Letrozole, the chemistry for Method-1 is summarized in Scheme-1.
[0004]
The Method-1 for synthesis of Letrozole as disclosed by Bowman et al. in
US 4,978,672 , and
US 5,352,795 and as summarized in Scheme-1, comprises reaction of alpha-bromo-4 tolunitrile or 4-bromomethyl benzonitrile (II) with 1H-1, 2,4-triazole (III), in a mixture of chloroform and acetonitrile as solvent at reflux temperature for 15 hours to give 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV), which on reaction with 4-flurobenzonitrile (VI) in the presence of potassium t-butoxide and in N, N-dimethylformamaide, gives crude Letrozole (I), which is recrystallized from 95% ethanol or a mixture of ether and ethyl acetate to give pure Letrozole (I):
[0005]
As would be evident from Examples 9, 25, and 26 of
US 4,978,672 , and
US 5,352,795 , in the step reaction of alpha-bromo-4 tolunitrile or 4-bromomethyl benzonitrile (II) with 1H-1, 2,4-triazole (III), as per Method-1, Scheme-I, in addition to the desired 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV) an appreciable amount of isomeric 4-[1-(1,3,4-triazolyl) methyl]-benzonitrile (V) is also formed in the reaction, which necessitates separation of the two isomers by column chromatography, subsequent to which the separated pure 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV) is reacted with 4-flurobenzonitrile (VI) to give Letrozole. Example 25 of
US 4,978,672 , and
US 5,352,795 further report that Letrozole obtained after recrystalization from 95% ethanol has a melting point of 181° - 183°C, while Example 26 reports that Letrozole obtained after recrystalization from a mixture of ether and ethyl acetate has a melting point of 184°- 185° C.
[0006]
The major disadvantage and limitation of the Method-1 disclosed in
US 4,978,672 , and
US 5,352,795 is that it leads to formation of appreciable amounts of the unwanted isomer i.e. 4-[1-(1,3,4-triazolyl) methyl]-benzonitrile (V), calling for tedious chromatographic techniques for its separation from the desired isomer i.e. 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV), which is expected to result in considerable loss and low yield of the desired isomer. Such a method, obviously, cannot be expected to be economically or commercially viable. Further, nowhere in the Specifications and Experimental Descriptions of
US 4,978,672 , and
US 5,352,795 there is any mention about the yield and purity of Letrozole obtained by the method described therein.
[0007]
The second method, Method-2, reported by Bowman et al. in
US 4,978,672 , and
US 5,352,795 is summarized in Scheme-II, which comprises of reaction of N-tert.butyl-4-bromo benzamide (1) with n-butyllithium and ethyl formate to give Bis- (4-N-tert.butyl carbamoylphenyl) methanol (2), which on reaction with thionyl chloride gives 4-(alpha-chloro-4'cyanobenzyl)benzonitrile (3). Reaction of 4-(alpha-chloro-4'cyanobenzyl) benzonitrile (3) with 1H-1,2,4-triazole (III) gives Letrozole (I).
[0008]
The major disadvantage and limitation of the Method-2 disclosed in
US 4,978,672 , and
US 5,352,795 , as evident from Examples 3, 5 and 28, described therein, is that first of all it utilizes corrosive and hazardous n-butyllithium and thionyl chloride; which require special storage, handling and disposal as well as calls for cryogenic temperatures of -60° C and higher temperatures of about 160° C, which collectively renders the method unsafe and industrially and commercially not of particular viability. Further, as in the case of Method-1, nowhere in the Specifications and Experimental Descriptions of
US 4,978,672 , and
US 5,352,795 there is any mention about the yield and purity of Letrozole obtained by the Method-2 described therein. Furthermore, the reaction of 4-(alpha-chloro-4'cyanobenzyl) benzonitrile (3) with 1,2,4-triazole (III) would most likely result in formation of the corresponding isomer along with the desired Letrozole, which would involve tedious purification techniques for its separation.
[0009]
[0010]
2.
Wadhwa et al. in US 2005/0209294 A1 , recite a method for synthesis of the intermediate 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV), comprising reaction of alpha-bromo-4 tolunitrile or 4-bromomethyl benzonitrile (II) with a salt of 1H-1,2,4-triazole, preferably an alkali metal salt of 1H-1,2,4-triazole (4), in a suitable solvent at a temperature of between 10° to 15° C, followed by crystallization of the isolated product. The chemistry is summarized in Scheme-III.
[0011]
Wadhwa et al. in US 2005/0209294 A1 , while stating that the method disclosed by Bowman et al. in
US 4,978,672 , and
US 5,352,795 is not selective in that it produces the undesired isomeric 4-[1-(1,3,4-triazolyl) methyl]-benzonitrile (V) in about 50%, which as mentioned hereinbefore requires tedious chromatographic separation techniques for its removal, emphasize that by virtue of utilization of an alkali metal salt of 1H-1, 2,4-triazole (4), the desired 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV) is obtained in >96% selectivity, thereby circumventing the utilization of tedious chromatographic techniques for its purification. Wadhwa et al., further state that the said intermediate i.e. 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV), obtained by their method can be converted to Letrozole of US Pharmacopoeial Quality, through conventional procedure.
[0012]
While the method disclosed by
Wadhwa et al. in US 2005/0209294 A1 , reportedly affords the intermediate 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV) in >96% selectivity and further, reportedly does away with chromatographic techniques in its isolation, however, the entire Specification and the Experimental Description given in Example-1 therein, is silent about the actual yield and purity of not only the intermediate 4-[1-(1,2,4-triazolyl) methyl]-benzonitrite (IV) but also that of Letrozole obtained by the method. The industrial or commercial viability of the method, therefore, cannot be commented, in view of insufficient disclosure.
[0013]
3.
Kompella et al. in WO 2005/047269 A1 , disclose a method for separation of the Letrozole precursor, 4-[1-(1,2,4-triazolyl)methyl]-benzonitrile (IV) from its isomer, 4-[1-(1,3,4-triazolyl) methyl]-benzonitrile (V), comprising treating a solution of the mixture of the two isomeric compounds (IV) and (V) in dichloromethane or chloroform with isopropylalcohol hydrochloride, followed by addition of isopropyl ether, wherein the hydrochloride salt of the undesired 4-[1-(1,3,4-triazolyl) methyl]-benzonitrile (V) precipitates out, which is removed by filtration. Basification of the filtrate, followed by evaporation of solvent and isolation of the residue from hexane or petroleum ether affords the desired 4-[1-(1,2,4-triazolyl)methyl]-benzonitrile (IV). The method is summarized in Scheme-IV.
[0014]
The required isomer is obtained in 47-61 % yield and a purity of about 99%.
[0015]
4. In another variant of the Method-1 of Bowman et al., an improved regiospecific method disclosed by
Patel et al. in US 2006/0128775 A1 for synthesis of Letrozole is summarized in Scheme-V.
[0016]
The method disclosed by
Patel et al. in US 2006/0128775 A1 utilizes 4-amino-1, 2,4-triazole (5), instead of 1H-1, 2,4-triazole (III) or an alkali metal salt of 1H-1, 2,4-triazole (4), as utilized by
Bowman et al. in US 4,978,672 , and
US 5,352,795 and
Wadhwa et al. in US 2005/0209294 A1respectively, for reaction with alpha-bromo-4 tolunitrile or 4-bromomethyl benzonitrile (II) to give 4-[(4-amino-1,2,4-triazolium-1-yl)methyl]benzonitrile bromide (6), which on diazotisation leads to the required intermediate, 4-[1-(1,2,4-triazolyl)methyl]-benzonitrile (IV), further reaction of which with 4-flurobenzonitrile (VI) gives crude Letrozole, which is recrystallized from polar or non-polar solvents to give pure Letrozole (I).
[0017]
The method of
Patel et al. in US 2006/0128775 A1 , in the first place provides an elegant regiospecific synthesis of Letrozole in that it like the method of
Wadhwa et al. in US 2005/0209294 A1 , minimizes the formation of the undesired isomeric 4-[1-(1,3,4-triazolyl) methyl]-benzonitrile (V) and also does away with tedious chromatographic separation techniques.
[0018]
The method of
Patel et al. in US 2006/0128775 A1 , albeit, as evident from Example-1, described therein, reportedly gives Letrozole of 99.90% HPLC purity, however, gives Letrozole of the said purity only in an overall yield of 34%, which renders it of not being an particularly economic process. Secondly, the method comprises of an additional step of deamination of the intermediate compound (6), which in turn calls for a diazotization step, through utilization of sodium nitrite, which is hazardous and explosive, more suitable to small scale preparations rather than industrial manufacture. The method, hence, might not be particularly amenable for industrial scale-up and manufacture.
[0019]
5.
MacDonald et al. in US 2007/0066831 A1 , report another variant of the methods disclosed by
Bowman et al. in US 4,978,672 , and
US 5,352,795 and
Wadhwa et al. in US 2005/0209294 A1 in that the said method, as summarized in Scheme-VI comprises:
- a) Reaction of alpha-bromo-4 tolunitrile or 4-bromomethyl benzonitrile (II) with an alkali metal salt of 1H-1,2,4-triazole (4), in presence of a solvent selected from the group consisting of diemthylacetamide, N-methyl-2-pyrrolididone, or a mixture thereof, at a temperature of about -20° to 0°C to give 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV);
- b) Extracting the impurities form intermediate compound (IV), in a two phase system, comprising an aqueous phase and a water-immiscible phase; and
- c) Reacting compound (IV) with 4-flurobenzonitrile (VI), in presence of a solvent selected from the group consisting of dimethylformamide, diemthylacetamide, N-methyl-2-pyrrolididone, and tetrahydrofuran or a mixture thereof and a base selected from sodium bis(trimethylsilyl)amide, hexyl lithium, butyl lithium, lithium didsopropylamide, alkoxide or mixtures thereof.
[0020]
US 2007/0066831 A1 further, states that the steps (a) and (b) could be combined together resulting in a one-pot synthesis of Letrozole.
[0021]
In the first place, it might be mentioned herein that the chemistry disclosed by
Macdonald et al. in US 2007/0066831 A1 is a nominal variation of the method disclosed by
Wadhwa et al. in US 2005/0209294 A1 , in that uses specific solvents such as diemthylacetamide, and N-methyl-2-pyrrolididone for formation of compound (IV) and again utilizes the same solvents for obtaining Letrozole from compound (IV), in addition to use of specific lithium containing bases, most of which are hazardous and expensive, requiring special precautions during storage, handling and disposal.
[0022]
6. In yet another variation,
Radhakrishnan et al. in WO 2007/039912 provide a method for synthesis of Letrozole, as summarized in Scheme-VII, which is a one-pot synthesis comprising reaction of compounds (II) and (4) to give compound (IV), which without isolation and on further reaction with compound (VI) gives Letrozole.
[0023]
The major disadvantage with the method is that is still does not obliterate the use of chromatographic separation/purification of Letrozole.
[0024]
7.
Haider et al. in WO 2007/054964 A2 provide an improvement, as summarized in Scheme- VIII, over Method-1 disclosed by
Bowman et al. in US 4,978,672 and
US 5,352.795 . in that the improvement comprises of selective removal of the isomeric 4-[1-(1,3,4-triazolyl)methyl]-benzonitrile (V), formed in the reaction of compound (II) and (III) in isopropanol as solvent, through a method of extraction, which provides the desired 4-[1-(1,2,4-triazolyl)methyl]-benzonitrile (IV), of >99% purity, and relatively free of the isomeric impurity (V).
[0025]
The method of extraction, as taught by
Haider et al. in WO 2007/054964 A2 comprises repeated extraction of the reaction medium containing mixture of the desired 4-[1-(1,2,4-triazolyl)methyl]-benzonitrile (IV) and the undesired 4-[1-(1,3,4-triazolyl)methyl]-benzonitrile (V) with water and a water-immiscible solvent to afford the pure 4-[1-(1,2,4-triazolyl)methyl]-benzonitrile (IV) in the organic phase, which is then further converted to Letrozole (I) of >99% purity by conventional methods. Haider et al. also teach a process for conversion of the mixture of the desired 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV) and the undesired 4-[1-(1,3,4-triazolyl)methyl]-benzonitrile (V) to Letrozole, from which the isomeric form of Letrozole i.e. Isoletrozole (9) so formed is removed by repeated crystallization to afford Letrozole (I) of >99% purity. -
[0026]
It might be noted that the method of Haider et al., primarily is one for purification of the intermediate 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV) as well as Letrozole (I) for removal of the corresponding isomeric impurities and as such does not provide any inputs for controlling or minimization of the formation of the isomeric 4-(1-(1,3,4-triazolyl)methyl]-benzonitrile (V) in the reaction. Secondly, the method of extraction as well as purification taught by Haider et al. is tedious, comprising multiple extractions, with multiple solvents and this coupled with the fact that it does not provide any improvement in controlling or minimization of the formation of the isomeric 4-[1-(1,3,4-triazolyl)methyl]-benzonitrile (V) in the reaction, leads to significant losses, thereby resulting in rather low yields of Letrozole (I). The method, therefore, is not of commercial significance.
[0027]
8.
Pizzocaro et al. in WO 2007/090464 A1 , a process for preparation of Letrozole (I), as summarized in Scheme-IX, characterized in that it teaches either simultaneous addition of a solution of 4-[1-(1,2,4-triazolyl) methyll-benzonitrile (IV) and a solution of 4-fluorobenzonitrile (VI) in an aprotic dipolar solvent to a solution of an alkali metal alkoxide in the same aprotic dipolar solvent or addition of an unique solution in an aprotic dipolar solvent comprising of compounds (IV) and (VI) to aprotic dipolar solvent, and reacting at a temperature of between -20 to + 40° C.
[0028]
The method of Pizzocaro et al., in addition to involving adherence to several critical parameters like temperature, flow rate, etc. moreover, does not provide any details of the yields and purity of Letrozole, obtained by the methods described therein.
[0029]
9.
Srinivas et al. WO 2007/107733 A1 recite a further variation of Method-1 disclosed by et al. in
US 4,978,672 and
US 5,352,795 , for synthesis of Letrozole, substantially free from its isomeric impurity, which is summarized in Scheme-X. The method comprises reacting 4-bromomethylbenzonitrile (II), with 1H 1,2,4-triazole (III) in an organic solvent in presence of cesium carbonate and precipitation of 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV), thus formed from the reaction medium using a suitable organic solvent. The intermediate (IV) is further converted to Letrozole by reaction with 4-fluorobenzonitrile (VI) in presence of an organic solvent and silicon amine, which are lithium, sodium, or potassium disilazanes or monosilazane.
[0030]
The method utilizes sensitive and expensive silicon compounds like lithium hexamethyldisilazane, which requires highly controlled reaction conditions.
[0031]
10.
Hasson et al. in US 2007/0112203 A1 , provide a method, as summarized in Scheme-XI, for purification of a mixture containing Letrozole (I) and its isomeric impurity i.e. Isoletrozole (IX), which is an extension of Method-2 disclosed by
Bowman et al. in US 4,978,672 and
US 5,352,795 . The method takes advantage of the rapid oxidation of Isoletrozole (9) to 4,4'-dicyclobenzophenone (10), in comparison to Letrozole (I), the oxidized compound (10), being easily separable from Letrozole, can be removed by crystallization, affording pure Letrozole. The Letrozole product, in turn is prepared by Method-2 disclosed by
Bowman et al. in US 4,978,672 and
US 5,352,795 .
[0032]
From the Enabling Disclosures of
Hasson et al. in US 2007/0112203 A1 , it could be seen that the method of oxidative purification of Letrozole, does not provide the said Letrozole, free of the Isoletrozole impurity (IX), directly and in fact, about 1 to 4% of Isoletrozole (IX) remains in the product, which is further removed by successive crystallizations to provide Letrozole (I) of 99.9% purity.
It is also noted that Letrozole to some extent also undergoes oxidation, albeit slowly, resulting in formation of additional impurities. Removal of such impurities, coupled with the task of removal of Isoletrozole (IX) and 4,4-dicyclobenzophenone (10) results in significant yield loss, rendering the method not particularly attractive, economically.
[0033]
[0034]
The method of Palle et al. comprises reacting 4,4-(hydroxymethylene)bis benzonitrile (12), in turn obtained from 4,4-dibromobenzophenone (11), with p-toluenesulfonyl chloride to give the corresponding p-tolenesulfonate (13), which on reaction with 1H 1,2,4-triazole (III), gives crude Letrozole, which is further purified by successive chromatography and crystallization.
[0035]
The yield of the p-tolenesulfonate (13), in the key step is only 21%, indicative of formation of large amount of impurities in the said step. Further, the overall yield of Letrozole obtained by the method is only about 14%, which would render the method not viable commercially.
[0036]
[0037]
US 2007/0112202 A1 reports synthesis of Letrozole by the abovementioned method in 54-56% yield and having a HPLC purity 99.4%, which may not suit Pharmacopoeial standards, which suggests that the product obtained requires further purification, which, incidentally, is acknowledged by Friedman et al., who state that single purification using various solvents does not give Letrozole of acceptable purity, and hence multiple purifications are required to achieve the same. Needless to mention, this would result in significant loss of the precious product. Further, the novelty and inventiveness of the method is in question, since
Bowman et al. in US 4,978,672 and
US 5,352,795 have disclosed the same chemistry earlier.
[0038]
[0039]
The method is lengthy and the reported overall yield of Letrozole appears to be only 9-11%.
.................................................
The process for preparation of 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile hydrochloride of formula (VII) and Letrozole of formula (I), both having a purity of ≥99% as per the present invention is schematically represented in Scheme-XV.
Reference Example - 3 Preparation of 4-[1-(4-cyanophenyl)-1-(1,2,4-trinzol-1yl)methyl]benzonitrile (Letrozole, I)
[0101]
To a mixture of potassium tertiarybutoxide (635.92 gm; 5.66 mol) and N,N-dimethylformamide (3.75 Lt), under an atmosphere of nitrogen and cooled to a temperature of -20° to -25°C, was added 4-(1H-1,2,4-triazol-1-ylmethyl)benzonitrile hydrochloride (VII, as obtained in Reference Examples 1 or 2; 250 gm; 1.13 mol) within 5 minutes and was stirred for 60 minutes at -20°C to -25°C. To the mixture was added 4-fluoro benzonitrile (VI, 150.9 gm; 1.24 mol) within 5 minutes and the mass agitated for an hour at-20°C to -25°C. After completion of the reaction, pH of the mixture was adjusted to between 6.0 to 6.5 by addition of 50% aqueous hydrochloric acid, maintaining the temperature between -20°C to 0°C. After the addition of the hydrochloric acid solution, the reaction mass was stirred for additional 30 minutes and filtered. To the filtrate was added ethyl acetate and water and the ethyl acetate layer was separated and dried over anhydrous sodium sulfate. The solvent was evaporated under vacuum to give a residual solid amounting to 179 gm (55%) of Letrozole (I), having a purity of 83%.
[0102]
The solid was chromatogaphed over silica gel (60-120 mesh) using n-Hexane and ethyl acetate as eluent to give Letrozole (100.5 gm; 56%), having a purity of 99%.
[0103]
The material (100 gm) was further dissolved in ethyl acetate (1.6 Lt) at 70° to 75°C, and the solution was filtered hot. The filtrate was evaporated under vacuum till the volume was between 200 to 220 ml. The solution was cooled to 0° to 5°C for 4 hours, and the solid filtered, washed with cold ethyl acetate and dried to give Letrozole (I, 95 gm; 95%), having a purity of 99.6%.
Example - 3 Preparation of 4-[1-(4-cyanophenyl)-1-(1,2,4-triazol-1-yl)methyl]benzonitrile (Letrozole, I)
[0104]
To a mixture of potassium tertiarybutoxide (635.92 gm; 5.66 mol) and N,N-dimethylformamide (3.75 Lt). under an atmosphere of nitrogen and cooled to a temperature of -20° to -25°C, was added 4-(1H-1,2,4-triazol-1-ylmethyl)benzonitrile hydrochloride (VII, as obtained in Examples 1 or 2; 250 gm; 1.13 mol) within 5 minutes and was stirred for 60 minutes at -20°C to -25°C. To the mixture was added 4-fluoro benzonitrile (VI, 150.9 gm; 1.24 mol) within 5 minutes and the mass agitated for an hour at -20°C to -25°C. After completion of the reaction, pH of the mixture, was adjusted to between 6.0 to 6.5 by addition of 50% aqueous hydrochloric acid, maintaining the temperature between -20°C to 0°C. After the addition of the hydrochloric acid solution, the reaction mass was stirred for additional 30 minutes and filtered. To the filtrate was added ethyl acetate and water and the ethyl acetate layer was separated and dried over anhydrous sodium sulfate. The solvent was evaporated under vacuum to give a residual solid amounting to 244 gm (75%) of Letrozole (I), having a purity of 99%.
[0105]
The material (244 gm) was further dissolved in ethyl acetate (500 ml) at 70° to 75°C, and the solution was filtered hot. The filtrate was cooled to 0° to 5°C for 4 hours, and the solid filtered, washed with cold ethyl acetate and dried to give Letrozole (I, 221 gm; 98.6%), having a purity of 99.7%.
..................................................
Reference Example - 3 Preparation of4-ll-(4-cyanophenyl)-l-(l,2,4-triazol-l-yl)methyl]benzonitrile (Letrozole, I)
To a mixture of potassium tertiarybutoxide (635.92 gm; 5.66 mol) and N5N- dimethylformamide (3.75 Lt), under an atmosphere of nitrogen and cooled to a temperature of -20° to -25 °C, was added 4-(lH-l,2,4-triazol-l-ylmethyl)benzonitrile hydrochloride (VII, as obtained in Reference Examples 1 or 2; 250 gm; 1.13 mol) within 5 minutes and was stirred for 60 minutes at -20°C to -25°C. To the mixture was added 4-fluoro benzonitrile (VI, 150.9 gm; 1.24 mol) within 5 minutes and the mass agitated for an hour at - 20°C to -25°C. After completion of the reaction, pH of the mixture was adjusted to between 6.0 to 6.5 by addition of 50% aqueous hydrochloric acid, maintaining the temperature between -200C to 0°C. After the addition of the hydrochloric acid solution, the reaction mass was stirred for additional 30 minutes and filtered. To the filtrate was added ethyl acetate and water and the ethyl acetate layer was separated and dried over anhydrous sodium sulfate. The solvent was evaporated under vacuum to give a residual solid amounting to 179 gm (55%) of Letrozole (I), having a purity of 83%.
The solid was chromatogaphed over silica gel (60-120 mesh) using n-Hexane and ethyl acetate as eluent to give Letrozole (100.5 gm; 56%), having a purity of 99%.
The material (100 gm) was further dissolved in ethyl acetate (1.6 Lt) at 70° to 75°C, and the solution was filtered hot. The filtrate was evaporated under vacuum till the volume was between 200 to 220 ml. The solution was cooled to 0° to 5°C for 4 hours, and the solid filtered, washed with cold ethyl acetate and dried to give Letrozole (I, 95 gm; 95%), having a purity of 99.6%. Example - 3 Preparation of4-[l-(4-cyanophenyl)-l-(l,2,4-triazol-l-yl)methyl]benzonitrile (Letrozole, I)
To a mixture of potassium tertiarybutoxide (635.92 gm; 5.66 mol) and N,N- dimethylformamide (3.75 Lt). under an atmosphere of nitrogen and cooled to a temperature of -20° to -25°C, was added 4-(l H-l ,2,4-triazol-l -ylmethyl)benzonitrile hydrochloride (VII. as obtained in Examples 1 or 2; 250 gm; 1.13 mol) within 5 minutes and was stirred for 60 minutes at -20°C to -25°C. To the mixture was added 4-fluoro benzonitrile (VI, 150.9 gm; 1.24 mol) within 5 minutes and the mass agitated for an hour at -20°C to -25°C. After completion of the reaction, pH of the mixtureJwas adjusted to between 6.0 to 6.5 by addition of 50% aqueous hydrochloric acid, maintaining the temperature between -20°C to 0°C. After the addition of the hydrochloric acid solution, the reaction mass was stirred for additional 30 minutes and filtered. To the filtrate was added ethyl acetate and water and the ethyl acetate layer was separated and dried over anhydrous sodium sulfate. The solvent was evaporated under vacuum to give a residual solid amounting to 244 gm (75%) of Letrozole (I), having a purity of 99%.
The material (244 gm) was further dissolved in ethyl acetate (500 ml) at 70° to 75°C, and the solution was filtered hot. The filtrate was cooled to 0° to 5°C for 4 hours, and the solid filtered, washed with cold ethyl acetate and dried to give Letrozole (I, 221 gm; 98.6%), having a purity of 99.7%.
..............................
Aromatase is an enzyme, which effects aromatisation of ring A in the metabolic formation of various steroid hormones. Various cancers, for example, breast cancer is dependent upon circulating steroid hormones, which have an aromatic ring A. Such cancers can be treated by removing the source of ring A aromatised steroid hormones, for example by the combination of oophorectomy and adrenalectomy. An alternative way of obtaining the same effect is by administering a chemical compound, which inhibits the aromatisation of the steroid ring A.
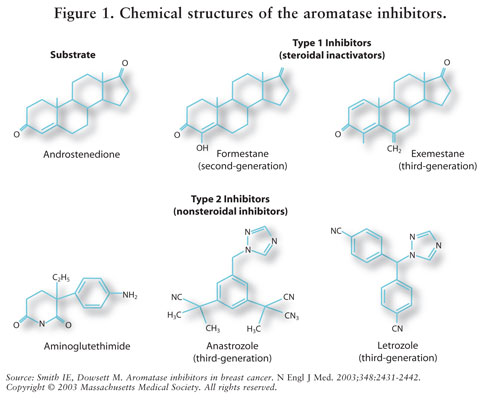
Letrozole is a non-steroidal antineoplastic, claimed to inhibit the aromatase (oestrogen synthase) activity. It is useful in the treatment of advanced breast cancer in postmenopausal women.
The growth of some cancers of the breast are stimulated or maintained by estrogens. Treatment of breast cancer thought to be hormonally responsive (i.e., estrogen and/or progesterone receptor positive or receptor unknown) has included a variety of efforts to decrease estrogen levels (ovariectomy, adrenalectomy, hypophysectomy) or inhibit estrogen effects (antiestrogens and progestational agents). These interventions lead to decreased tumor mass or delayed progression of tumor growth in some women.
In postmenopausal women, estrogens are mainly derived from the action of the aromatase enzyme, which converts adrenal androgens (primarily androstenedioήe and testosterone) to estrone and estradiol. The suppression of estrogen biosynthesis in peripheral tissues and in the cancer tissue itself can therefore be achieved by specifically inhibiting the aromatase enzyme.
Letrozole is a nonsteroidal competitive inhibitor of the aromatase enzyme system; it inhibits the conversion of androgens to estrogens. In adult tumor bearing females, Letrozole is as effective as ovariectomy in reducing uterine weight, elevating serum LH, and causing the regression of estrogen-dependent tumors. In contrast to ovariectomy, treatment with Letrozole does not lead to an increase in serum FSH. Letrozole selectively inhibits gonadal steroidogenesis but has no significant effect on adrenal mineralocorticoid or glucocorticoid synthesis. l Letrozole inhibits the aromatase enzyme by competitively binding to the heme of the cytochrome P450 subunit of the enzyme, resulting in a reduction of estrogen biosynthesis in all tissues. Treatment of women with Letrozole significantly lowers serum estrone, estradiol and estrone sulfate and has not been shown to significantly affect adrenal corticosteroid synthesis, aldosterone synthesis, or synthesis of thyroid hormones. Description of prior art
Synthesis of Letrozole is reported in US Patent No. 4,978,672 and EP 236,940. In the above patents the synthesis of Letrozole starts with 4-bromomethylbenzonitrile (1), which undergoes condensation with 1,2,4-triazole (2) to form 4-[(l,2,4-triazol-l- yl)methyl]benzonitrile (3) as an intermediate. The compound of structural formula (3) is purified by column chromatography to remove 4-[(l,3,4-triazol-l- yl)methyl]benzonitrile (4) and followed by reaction with 4-fluorobenzonitrile (5) to
SCHEME - 1
afford Letrozole (6).
In the above process, the undesired intermediate 4-[(l,3,4-triazol-l- yl)methyl]benzonitrile (4) is formed during the course of the preparation 4-[(l,2,4- triazol-l-yl)methyl]benzonitrile (3) in 10%w/w to 30%w/w. The undesired impurity 4- [(l,3,4-triazol-l-yl)methyl]benzonitrile (4) present with 4-[(l,2,4-triazol-l- yl)methyl]benzonitrile (3), further on reaction with 4-fluorobenzonitrile (5) leads to the formation of another impurity 4-[l-(4-cyanophenyl)-l-(l,3,4-triazol-l- yl)methyl]benzonitrile (7) in approximately same ratio.
To control the formation of impurity of structural formula (7), it is required to make intermediate of structural formula (3) in its pure form. The separation of desired compound from isomeric impurities is of great importance. In basic patents US 4,978,672 and EP 236,940; chromatographic technique is used to isolate intermediate (3) from its mixture with regioisomer (4). Chromatography has its own limitations on commercial scale; it is an expensive and time consuming operation at plant scale, which also consumes lots of solvent and is hazardous for environment.
To overcome the above problems, purification of intermediate (3) is reported in PCT application WO 2005/047269 via the hydrochloride salt formation of the mixture of product (3) along with regioisomer (4). Selective crystallisation of regioisomer as hydrochloride using approximately 8.5 volume diisopropyl ether, filtering the resultant and then isolation of the intermediate (3) as pure product from the filtrate in approximately 60% overall yield. Finally washing the product with hexane or petroleum ether. In above PCT application, highly flammable solvents like diisopropyl ether, hexane and petroleum ether are used in process, which require high level of precautions and are never safe to handle on plant scale.

Another process reported in PCT application WO 2004/076409, discloses the different route to prepare the pure intermediate (3). The said patent discloses a reaction of 4-bromomethylbenzonitrile (1) with 4-amino-l,2,4-triazole (8) to give quaternary ammonium salt (9), which undergoes diazotisation reaction to give 4-[(l,2,4-triazol-l- yl)methyl]benzonitrile (3) in approximately 59% molar yield. The process is complicated and involves lengthy steps and tedious operations. Objects of the invention
It is therefore, an important object of the present invention to provide a process for the preparation of Letrozole which avoids the use of highly flammable solvents and is safe and smooth.. Summary of the invention
To overcome the problems in the use of highly flammable solvents, complicated and lengthy steps and tedious operations; we have opted a simple and novel process for the purification of Letrozole intermediate (3), which is free from its regioisomer (4) and other related impurities.
Purification of intermediate (3) to remove its regioisomer (4) using crystallization method to achieve desired level of purity is unsuccessful. We have planned to go for extraction of intermediate (3) selectively from the mixture with regioisomer (4) in aqueous layer using a suitable solvent.
LETROZOLE
In order to obtain the pure Letrozole (6), we have planned to get intermediate (3) in its pure form and free from its regioisomer (4). For the said purpose, we have used solvent extraction method using suitable solvent system and selectively extract the desired intermediate 4-[(l,2,4-triazol-l-yl)methyl]benzonitrile (3), from a mixture with regioisomer 4-[(l,3,4-triazol-l-yl)methyl]benzonitrile (4). The control of the regioisomer at intermediate level leads its control at the final stage. Therefore, in an embodiment, the present invention relates to Letrozole (6) with its regioisomer 4-[l-(4-cyanophenyl)-l-(l,3,4-triazol-l-yl)methyl]benzonitrile (7), preferably, less than 0.3%w/w, more preferably, less than 0.1%w/w and most preferably, below the quantitation limit.
In another feature, the present invention provides an improved process for the preparation of Letrozole with its regioisomer 4-[l-(4-cyanophenyl)-l-(l,3,4-triazol-l- yl)methyl]benzonitrile (7), preferably, less than 0.3%w/w, more preferably, less tjian 0.1%w/w and most preferably, below the quantitation limit.
In another feature, the present invention provides 4-[(l,2,4-triazol-l- yl)methyl]benzonitrile (3) with its regioisomer 4-[(l,3,4-triazol-l- yl)methyl]benzonitrile (4), preferably, less than 0.3%w/w, more preferably, less than 0.1%w/w and most preferably, below the quantitation limit.
SCHEME - 4
(5)
In yet another feature, the present invention provides an improved process for the preparation of 4-[(l,2,4-triazol-l-yl)methyl]benzonitrile (3) with its regioisomer A- [(l,3,4-triazol-l-yl)methyl]benzonitrile (4), preferably, less than 0.3%w/w, more preferably, less than 0.1%w/w and most preferably, below the quantitation limit.
In order to obtain Letrozole (6) in purer form and free from its regioisomer (7) and other related impurities; intermediate 4-[(l,2,4-triazol-l-yl)methyl]benzonitrile (3) is to be prepared in its pure form, free from its regioisomer 4-[(l,3,4-triazol-l- yl)methyl]benzonitrile (4) and other related impurities. Thus, the main aspect of the present invention relates to the preparation of Letrozole (6) with its regioisomer (7) preferably less than 0.3%, more preferably less than 0.1% and most preferably below quantitation limit. For this purpose intermediate 4-[(l,2,4-triazol-l- yl)methyl]benzonitrile (3) is required to be of the same purity level. Another aspect of the present invention relates to the preparation of 4-[(l,2,4-triazol-l- yl)methyl]benzonitrile (3) with its regioisomer 4-[(l,3,4-triazol-l- yl)methyl]benzonitrile (4) preferably less than 0.3%, more preferably less than 0.1% and most preferably below quantitation limit.

It has been also found that during the preparation of Letrozole intermediate A- [(l,2,4-triazol-l-yl)methyl]benzonitrile (3) another impurity is formed, which is characterized as the quaternary salt (10). To control the formation of this quaternary salt, mole ratio of 1,2,4-triazole is optimized preferably from 1.5 mole to 4.4 mole equivalents and more preferably to 3.0 mole equivalents with respect to A- bromomethylbenzonitrile (1). Thus, another aspect of the present invention relates to the preparation of Letrozole (6) with quaternary salt (10) preferably less than 0.1% and more preferably below quantitation limit. Another important aspect of the present invention relates to the preparation of 4-
[(l,2,4-triazol-l-yl)methyl]benzonitrile (3) in the pure form, free from its regioisomer 4-[(l,3,4-triazol-l-yl)methyl]benzonitrile (4). The purification of 4-[(l,2,4-triazol-l- yl)methyl]-benzonilrilc (3) takes place by its selective extraction from a mixture with its regioisomer 4-[(l,3,4-triazol-l-yl)methyl]benzonitrile (4) by using suitable solvents and/or mixture of solvents.
According to another aspect of the present invention Letrozole intermediate A- [(l,2,4-triazol-l-yl)methyl]benzonitrile (3) is prepared with its regioisomer 4-[(l,3,4- triazol-l-yl)methyl]benzonitrile (4) less than 30% and followed by the preparation of Letrozole enriched with its regioisomer (7), which is removed by using crystallisation method using suitable solvent system.
Example - 3
4-[(l,2,4-triazol-l-yl)methyl]benzonitrile (S) with a mixture of 4-[(l,3,4-triazol-l- yl)methyl]benzonitrile (4) To a 250 mL three neck R. B. Flask fitted with a reflux condenser and a thermometer pocket, isopropanol (37.5 mL), p-cyanobenzylbromide (25 g), 1,2,4- triazole (26.4 g) and potassium carbonate (52.8 g) were charged to the reaction mixture at RT with stirring. The reaction mixture was heated to 60-65 0C for 1.0 hr. The progress of reaction was monitored over TLC for the absence of p- cyanobenzylbromide. After completion, the reaction mixture was cooled down to RT and water (100 mL) was added to the reaction mixture and reaction mass was transferred to a one lit R. B. Flask containing water (275 mL). Cone. HCl (50 mL) was added very slowly to the reaction mass to adjust pH 7 - 8. The reaction mixture was extracted from dichloromethane (250 mL). Dichloromethane layer was distilled out at 50 0C giving 21.0 gm viscous oily residue. The residue is crystallized from a mixture of IPA: Cyclohexane (1:10) to give 18 g of 4-[(l,2,4-triazol-l-yl)methyl]benzonitrile (3) with a mixture of its regioisomer (4). HPLC Purity ~ 85%; Regioisomer ~ 13%. Example - 4 4-[l-(4-cyanophenyl)-l-(l,2,4-triazol-l-yl)methyl]benzonitrile (6) & 4-[l-(4- cyanophenyl)-l-(l,3,4-triazol-l-yl)methyl]benzonitrile (7)
In a 250 mL three neck flask, equipped with thermometer pocket, mechanical stirrer and a guard tube, THF (50 mL) was charged at room temperature. Potassium tert-butoxide (12.3 gm) is added in small portions in 30 minutes. The solution was cooled to -15 0C and a solution of product from example-3 (10 g) and p- fluorobenzonitrile (8.5 g) in THF (50 mL) is added very slowly to the reaction mixture in 4-5 hrs. Stir the reaction mixture at same temperature for 3 hrs. Progress of the reaction is monitored on TLC. Dichloromethane (200 mL) is added to the reaction mixture followed by the addition of acetic acid (7 g). Reaction mixture is added to another flask containing water (220 mL). pH of the reaction mixture is adjusted to 7-8 by addition of 5% sodium bicarbonate solution (180 mL). Dichloromethane layer is washed with water (200 mL), separated, filtered through hyfiow bed and distilled at a temperature below 50 0C. The residue obtained was crystallized from Isopropanol (20 mL) to get the solid product (4.9 g). HPLC Purity: 89.6%. Regioisomer: 7.41%. Example - 5
Removal of regioisomer (7) from Letrozole (6)
To a 250 mL R. B. Flask crude Letrozole (4.5 g) was charged in methanol (115 mL) and heated to 60 0C to dissolve completely and get clear solution. Methanol (approx. 100 mL) was distilled out and the solution was cooled to 25 - 30 0C, and was stirred for 2 hrs at this temperature. Solid product was filtered and washed with methanol (10 mL x 2) to get solid product, which was dried in vacuum to get 3.5 gm of product.
HPLC Purity: 96.33%, Regioisomer: 3.4%
Using the same purification method, desired purity of Letrozole had been achieved containing acceptable level of regioisomer (7).
Following the above purification from methanol repeatedly, the Letrozole may be prepared with the desired limit of its regioisomer (7).
References
- 003330 Letrozole
- Drugs.com: monograph for letrozole. It is also used for ovarian cancer patients after they have completed chemotherapy.
- Tulandi T, Martin J, Al-Fadhli R, et al. (June 2006). "Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate". Fertility and Sterility 85 (6): 1761–5. doi:10.1016/j.fertnstert.2006.03.014. PMID 16650422.
- Sinha, Kounteya (18 October 2011). "Finally, expert panel bans fertility drug Letrozole". The Times of India. Retrieved 14 November 2011.
- "House panel to govt: Punish those guilty of approving Letrozole". The Times of India. 10 April 2007. Retrieved 9 May 2012.
- Chen DT, Wynia MK, Moloney RM, Alexander GC (2009). "Physician knowledge of the FDA-approved indications of commonly prescribed drugs: results of a national survey". Pharmacoepidemiology and Drug Safety 18 (11): 1–7. doi:10.1002/pds.1825. PMID 19697444.
- "GMC | Good practice in prescribing medicines – guidance for doctors". Gmc-uk.org. 16 February 2007. Retrieved 21 November 2011.
- Vivian Chi Yan Lee, Ernest Hung Yu Ng, William Shu Biu Yeung, Pak Chung Ho (2011). "Misoprostol With or Without Letrozole Pretreatment for Termination of Pregnancy". Ob Gyn. 117 (2, Part 1): 317–323. doi:10.1097/AOG.0b013e3182073fbf.
- Santen, R. J.; Brodie, H.; Simpson, E. R.; Siiteri, P. K.; Brodie, A. (2009). "History of Aromatase: Saga of an Important Biological Mediator and Therapeutic Target". Endocrine Reviews 30 (4): 343–375. doi:10.1210/er.2008-0016. PMID 19389994. edit
- "Gynecomastia and Letrozole". GYNECOMASTIA-GYNO.COM: ...a resource for gynecomastia sufferers... 16 December 2008. Archived from the original on 26 June 2010. Retrieved 26 April 2012.
- Geneviève Patry, Keith Jarvi, Ethan D. Grober, Kirk C. Lo (August 2009). "Use of the aromatase inhibitor letrozole to treat male infertility". Fertility and Sterility 92 (2): 829.e1–829.e2. doi:10.1016/j.fertnstert.2009.05.014.
- R Eshet, G Maor, T Ben Ari, M Ben Eliezer, G Gat-Yablonski, M Phillip (2004). "The aromatase inhibitor letrozole increases epiphyseal growth plate height and tibial length in peripubertal male mice". Journal of Endocrinology 182 (1): 165–172. doi:10.1677/joe.0.1820165. PMID 15225141.
- Ping Zhou MD, Bina Shah MD, Kris Prasad PhD, Raphael David MD (2005). "Letrozole Significantly Improves Growth Potential in a Pubertal Boy With Growth Hormone Deficiency". Journal of the American Academy of Pediatrics 115 (2): 245–248. doi:10.1542/peds.2004-1536. PMID 15653791.
- Endometriosis ESHRE abstract
- Haberfeld, H, ed. (2009). Austria-Codex (in German) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 3-85200-196-X.
- Simpson ER (2003). "Sources of estrogen and their importance". The Journal of Steroid Biochemistry and Molecular Biology 86 (3–5): 225–30. doi:10.1016/S0960-0760(03)00360-1. PMID 14623515.
- Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer, the BIG 1–98 Collaborative Group, N Engl J Med, 361:766, 2009 Aug 20
- 32nd Annual San Antonio Breast Cancer Symposium
- Lang, M; Batzl, C; Furet, P; Bowman, R; Hausler, A; Bhatnagar, A (1993). "Structure-activity relationships and binding model of novel aromatase inhibitors". The Journal of Steroid Biochemistry and Molecular Biology 44 (4–6): 421–8. doi:10.1016/0960-0760(93)90245-R. PMID 8476755.
External links