 GMP......API PILOT PLANT
PRESENTATION
GMP......API PILOT PLANT
PRESENTATION
Pilot plant and scale-up techniques are both integral and critical to drug discovery and development process for new medicinal products. A major decision focuses on that point where the idea or process is advanced from a research oriented program targeted towards commercialization.
The speed of drug discovery has been accelerating at an exponential rate. The past two decades particular have witnessed amazing inventions and innovations in pharmaceutical research, resulting in the ability to produce new drugs faster than even before.
The new drug applications (NDAs) and abbreviated new drug applications (ANDA) are all-time high. The preparation of several clinical batches in the pilot plant provides its personnel with the opportunity to perfect and validate the process. Also different types of laboratories have been motivated to adopt new processes and technologies in an effort to stay at the forefront scientific innovation.
MY PRESENTATIONPharmaceutical pilot plants that can quickly numerous short-run production lines of multiple batches are essential for ensuring success in the clinical testing and bougainvilleas study phases. Drug formulation research time targets are met by having a well-designed facility with the appropriate equipment mix, to quickly move from the laboratory to the pilot plant scale 1. In pilot plant, a formula is transformed into a viable, robust product by the development of a reliable and practical method of manufacture that effects the orderly transition from laboratory to routine processing in a full scale production facility where as the scale up involves the designing of prototype using the data obtained from the pilot plant model.
Pilot plant studies must includes a close examination of formula to determine its ability to withstand batch-scale and process modifications; it must includes a review of range of relevant processing equipment also availability of raw materials meeting the specification of product and during the scale up efforts in the pilot plant production and process control are evaluated, validated and finalized.
In addition, appropriate records and reports issued to support Good Manufacturing Practices and to provide historical development of the production formulation, process, equipment train, and specifications
A manufacturer’s decision to scale up / scale down a process is ultimately rooted in the economics of the production process, i.e., in the cost of material, personnel, and equipment associated with the process and its control.
When developing technologies, there are a number of steps required between the initial concept and completion of the final production plant. These steps include the development of the commercial process, optimization of the process, scale-up from the bench to a pilot plant, and from the pilot plant to the full scale process. While the ultimate goal is to go directly from process optimization to full scale plant, the pilot plant is generally a necessary step. Reasons for this critical step include: understanding the potential waste streams, examination of macro-processes, process interactions, process variations, process controls, development of standard operating procedures, etc. The information developed at the pilot plant scale allows for a better understanding of the overall process including side processes. Therefore, this step helps to build the information base so that the technology can be permitted and safely implemented. Should be versatile pilot plant that is entirely GMP and facilitates the development of API’s in scalable, safe and environmentally friendly ways. The combination of facilities, experience and flexibility enable an integral Contract Manufacturing service ranging from laboratory to industrial scale; it should manufacture under regulation small amounts of high added value active substances or key intermediate products.
The combination of facilities, experience and flexibility enable an integral Contract Manufacturing service ranging from laboratory to industrial scale; it should manufacture under regulation small amounts of high added value active substances or key intermediate products.
 Product quality: Operations that depend on people for executing manual recipes are subject to human variability. How precisely are the operators following the recipe? Processes that are sensitive to variations in processing will result in quality variation. Full recipe automation that controls most of the critical processing operations provides very accurate, repeatable material processing. This leads to very highly consistent product quality.
Product quality: Operations that depend on people for executing manual recipes are subject to human variability. How precisely are the operators following the recipe? Processes that are sensitive to variations in processing will result in quality variation. Full recipe automation that controls most of the critical processing operations provides very accurate, repeatable material processing. This leads to very highly consistent product quality.
 Improved production: Many biotech processes have extremely long cycle times (some up to 6 months), and are very sensitive to processing conditions. It is not uncommon for batches to be lost for unexplained reasons after completing a large portion of the batch cycle time. The longer the batch cycle time and the more sensitive production is to processing conditions, the more batch automation is justified. Imagine losing a batch of very valuable product because the recipe was not precisely followed!
Process optimization: Increasing the product yield can be done by making small changes in processing conditions to improve the chemical conversions or biological growth conditions. Manual control offers a limited ability to finely implement small changes to processing conditions due to the inherent lack of precision in human control. Conversely, computers are very good at controlling conditions precisely. In addition, advanced control capabilities such as model predictive control can greatly improve process optimization. This results in higher product yield and lower production cost. This consideration is highly relevant to pilot plant facilities where part of the goal is to learn how to make the product.
Improved production: Many biotech processes have extremely long cycle times (some up to 6 months), and are very sensitive to processing conditions. It is not uncommon for batches to be lost for unexplained reasons after completing a large portion of the batch cycle time. The longer the batch cycle time and the more sensitive production is to processing conditions, the more batch automation is justified. Imagine losing a batch of very valuable product because the recipe was not precisely followed!
Process optimization: Increasing the product yield can be done by making small changes in processing conditions to improve the chemical conversions or biological growth conditions. Manual control offers a limited ability to finely implement small changes to processing conditions due to the inherent lack of precision in human control. Conversely, computers are very good at controlling conditions precisely. In addition, advanced control capabilities such as model predictive control can greatly improve process optimization. This results in higher product yield and lower production cost. This consideration is highly relevant to pilot plant facilities where part of the goal is to learn how to make the product.
 Recordkeeping: A multi-unit recipe control system is capable of collecting detailed records as to how a batch was made and relates all data to a single batch ID. Data of this nature can be very valuable for QA reporting, QA deviation investigations, and process analysis.
Safety: Operators spend less time exposed to chemicals when the process is fully automated as compared to manual control. Less exposure to the process generally results in a safer process.
A good batch historian should be able to collect records for a production run to include the following information: Product and recipe identification
User defined report parameters
Formulation data and relevant changes
Procedural element state changes (Operations, unit procedures, procedures)
Phase state changes
Operator changes
Operator prompts and responses
Operator comments
Equipment acquisitions and releases
Equipment relationships
Campaign creation data (recipe, formula values, equipment, etc.)
Campaign modifications
Campaign execution activity
Controller I/O subsystem events from the Continuous Historian
Process alarms
Process events
Device state changes.
Raw materials
Buildings and facilities. GMPs under the 21 Code of Federal Regulations (CFR) Part 211.42 state that buildings or areas used in the receiving, storage, and handling of raw materials should be of suitable size, construction and location to allow for the proper cleaning, maintenance, and operation (7). The common theme for this section of CFR Parts 210 and 211 is the prevention of errors and contamination. In principle, the requirements for buildings and facilities used in early phase manufacturing are not significantly different than those for later phases or even commercial production. However, there are some areas that are unique to early clinical trial manufacturing.
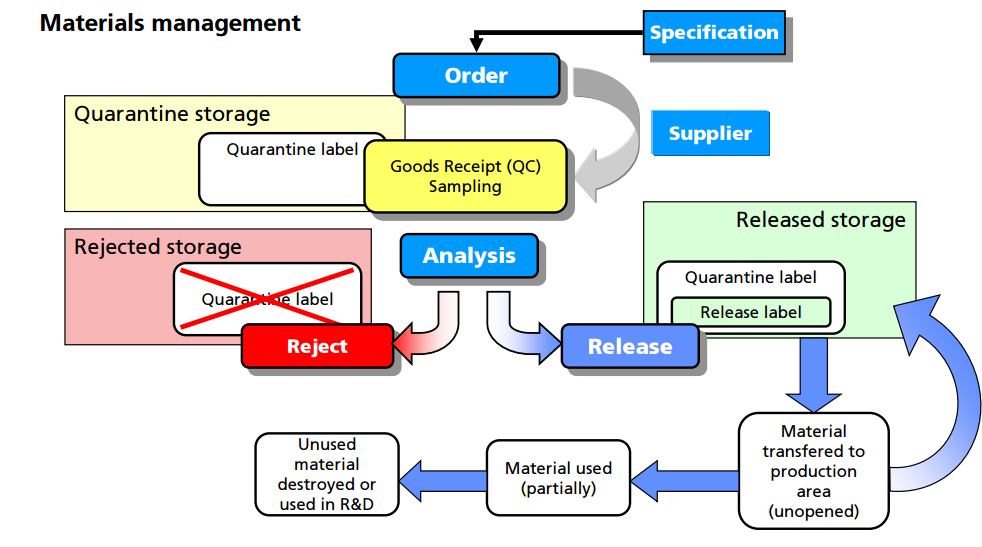
Control of materials. The CFR regulations under Part 211.80 provide good direction with respect to lot identification, inventory, receipt, storage, and destruction of materials (7). The clear intent is to ensure patient safety by establishing controls that prevent errors or cross-contamination and ensure traceability of components from receipt through clinical use. In general, the requirements for the control of materials are identical across all phases of development, so it is important to consider these requirements when designing a GMP facility within a laboratory setting.
Recordkeeping: A multi-unit recipe control system is capable of collecting detailed records as to how a batch was made and relates all data to a single batch ID. Data of this nature can be very valuable for QA reporting, QA deviation investigations, and process analysis.
Safety: Operators spend less time exposed to chemicals when the process is fully automated as compared to manual control. Less exposure to the process generally results in a safer process.
A good batch historian should be able to collect records for a production run to include the following information: Product and recipe identification
User defined report parameters
Formulation data and relevant changes
Procedural element state changes (Operations, unit procedures, procedures)
Phase state changes
Operator changes
Operator prompts and responses
Operator comments
Equipment acquisitions and releases
Equipment relationships
Campaign creation data (recipe, formula values, equipment, etc.)
Campaign modifications
Campaign execution activity
Controller I/O subsystem events from the Continuous Historian
Process alarms
Process events
Device state changes.
Raw materials
Buildings and facilities. GMPs under the 21 Code of Federal Regulations (CFR) Part 211.42 state that buildings or areas used in the receiving, storage, and handling of raw materials should be of suitable size, construction and location to allow for the proper cleaning, maintenance, and operation (7). The common theme for this section of CFR Parts 210 and 211 is the prevention of errors and contamination. In principle, the requirements for buildings and facilities used in early phase manufacturing are not significantly different than those for later phases or even commercial production. However, there are some areas that are unique to early clinical trial manufacturing.
Control of materials. The CFR regulations under Part 211.80 provide good direction with respect to lot identification, inventory, receipt, storage, and destruction of materials (7). The clear intent is to ensure patient safety by establishing controls that prevent errors or cross-contamination and ensure traceability of components from receipt through clinical use. In general, the requirements for the control of materials are identical across all phases of development, so it is important to consider these requirements when designing a GMP facility within a laboratory setting.
 For example, all materials must be assigned a unique lot number and have proper labeling. An inventory system must provide for tracking each lot of each component with a record for each use. Upon receipt, each lot should be visually examined for appropriate labeling and for evidence of tampering or contamination. Materials should be placed into quarantine or in the approved area or reject area with proper labeling to identify the material and prevent mix-ups with other materials in the storage area. Provision should be made for materials with special storage requirements (e.g., refrigeration, high security). The storage labeling should match the actual conditions that the material is being stored and should include expiry/retest dates for approved materials. Although such labeling is inconvenient for new materials where the expiration or retest date may change as more information is known, this enables personnel to be able to determine quickly whether a particular lot of a material is nearing or exceeding the expiration or retest date. General expiry/retest dates for common materials should be based on manufacturer's recommendation or the literature.
Finally, there are clear regulatory and environmental requirements for the destruction of expired or rejected materials. It is important to observe regional and international requirements regarding the use of animal sourced materials (12). It is recommended to use materials that are not animal sourced and that there be available certification by the raw material manufacturers that they contain no animal sourced materials. If animal sourced raw materials must be used, then certifications by the raw material manufacturers that they either originate from certified and approved (by regulatory bodies) sources for use in human pharmaceuticals, or that the material has been tested to the level required for acceptance by regulatory agencies (following US, EU, or Japanese guidelines, as applicable) is required.
For example, all materials must be assigned a unique lot number and have proper labeling. An inventory system must provide for tracking each lot of each component with a record for each use. Upon receipt, each lot should be visually examined for appropriate labeling and for evidence of tampering or contamination. Materials should be placed into quarantine or in the approved area or reject area with proper labeling to identify the material and prevent mix-ups with other materials in the storage area. Provision should be made for materials with special storage requirements (e.g., refrigeration, high security). The storage labeling should match the actual conditions that the material is being stored and should include expiry/retest dates for approved materials. Although such labeling is inconvenient for new materials where the expiration or retest date may change as more information is known, this enables personnel to be able to determine quickly whether a particular lot of a material is nearing or exceeding the expiration or retest date. General expiry/retest dates for common materials should be based on manufacturer's recommendation or the literature.
Finally, there are clear regulatory and environmental requirements for the destruction of expired or rejected materials. It is important to observe regional and international requirements regarding the use of animal sourced materials (12). It is recommended to use materials that are not animal sourced and that there be available certification by the raw material manufacturers that they contain no animal sourced materials. If animal sourced raw materials must be used, then certifications by the raw material manufacturers that they either originate from certified and approved (by regulatory bodies) sources for use in human pharmaceuticals, or that the material has been tested to the level required for acceptance by regulatory agencies (following US, EU, or Japanese guidelines, as applicable) is required.
Direct advantages for customers
- Shorter implementation time for product by determination of the product suitability as well as the necessary process cycle
- Optimized adjustment of the processing times in the production lines (trains) by relatively precise estimation of the drying times
- Definition of effective cleaning processes (CIP/WIP and SIP)
- Definition of the selection criteria based on the weighting of the customer, e.g.: drying time, quality (form of crystal, activity, etc.), cleanout, ability of CIP, price
An overview of further trials and test functions, that can be realized in the new pilot plant facility:
- Product tests for determination of suitability
- Scale-up tests as basis for the extrapolation on production batches regarding drying time, filling degree, crystalline transformation and grain spectrum
- Optimization of the process cycle
- Optimization of the machine
- Data acquisition and analysis
Case study 1
 Designed and equipped for the manufacturing of solid oral dosage forms, specialized in high-activity substances (cytostatic, cytotoxic, hormonal, hormone inhibitors). It has ancillary areas for the proper management of materials intended for clinical trials of new drugs.
Equipment:
Designed and equipped for the manufacturing of solid oral dosage forms, specialized in high-activity substances (cytostatic, cytotoxic, hormonal, hormone inhibitors). It has ancillary areas for the proper management of materials intended for clinical trials of new drugs.
Equipment:
- • Oystar Hüttlin Pilotmix 75T single-pot high shear mixer.
- • Container mixer, 60 and 200 liters containers.
- • Fette 1200iC rotary tablet press, fully equipped for formulation development and manufacturing.
- • Oystar Manesty XL Lab 01 tablet coater, with interchangeable drums.
- • IMA Clinipack blister machine.
- • Quadro Comill sieve
- • Capsunorm 2000 manual capsul filler
- • Sepha press-out deblister
- • Fette Gratex deduster
- • Process control laboratory: Dr. Schleuniger tablet hardness tester, Erweka tablet disintegration tester, Erweka friability tester, Mettler Toledo moisture content determination equipment.
CASE STUDY 2
OPERATION OF PILOT PLANT FOR CLINICAL LOTS OF BIOPHARMACEUTICALS http://www.peq.coppe.ufrj.br/biotec/presentations/Papamichael_RioDeJaneiro2009_secure.pdf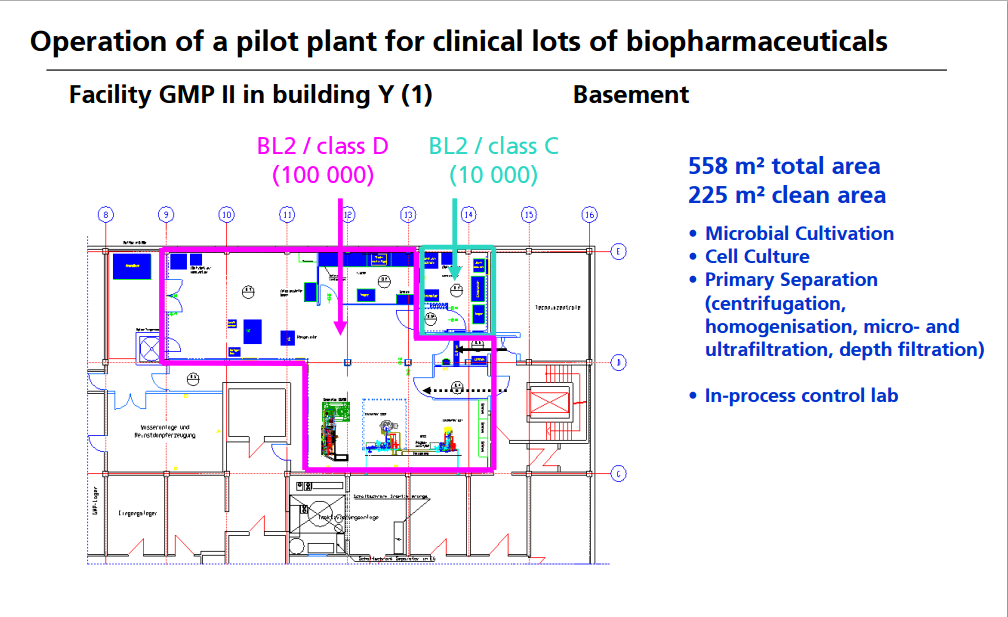


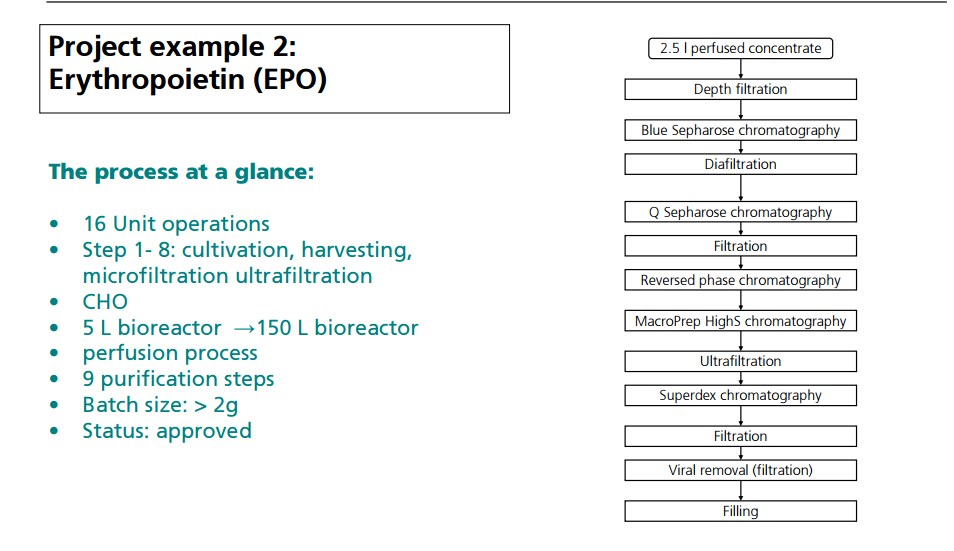
CASE STUDY 3
CASE STUDY 4
http://www.steroglass.it/doc_area_download/ita/process/20LT_PILOT_PLANT.pdf
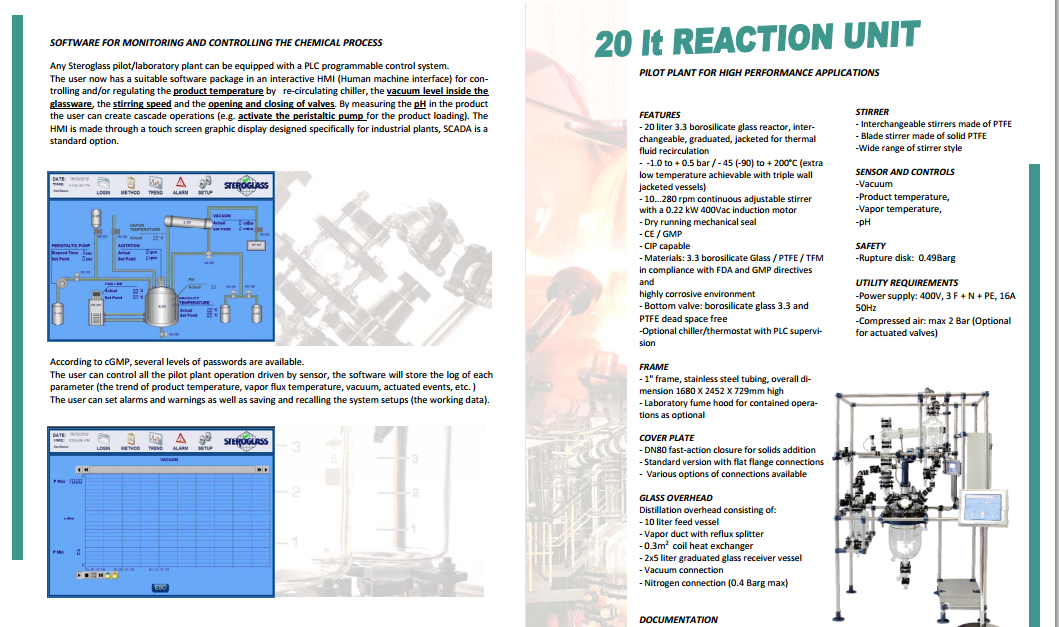
CASE STUDY 5
 Health Canada
http://www.hc-sc.gc.ca/dhp-mps/compli-conform/gmp-bpf/question/gmp-bpf-eng.php
The Good Manufacturing Practices questions and answers (GMP Q&A) presented below have been updated following the issuance of the "Good Manufacturing Practices Guidelines, 2009 Edition Version 2 (GUI-0001)".
This Q&A list will be updated on a regular basis.
Premises - C.02.004
Equipment - C.02.005
Personnel - C.02.006
Sanitation - C.02.007 & C.02.008
Raw Material Testing - C.02.009 & C.02.010
Manufacturing Control - C.02.011 & C.02.012
Quality Control Department - C.02.013, C.02.014 & C.02.015
Packaging Material Testing - C.02.016 & C.02.017
Finished Product Testing - C.02.018 & C.02.019
Records - C.02.020, C.02.021, C.02.022, C.02.023 & C.02.024
Samples - C.02.025 & C.02.026
Stability - C.02.027 & C.02.028
Sterile Products - C.02.029
Health Canada
http://www.hc-sc.gc.ca/dhp-mps/compli-conform/gmp-bpf/question/gmp-bpf-eng.php
The Good Manufacturing Practices questions and answers (GMP Q&A) presented below have been updated following the issuance of the "Good Manufacturing Practices Guidelines, 2009 Edition Version 2 (GUI-0001)".
This Q&A list will be updated on a regular basis.
Premises - C.02.004
Equipment - C.02.005
Personnel - C.02.006
Sanitation - C.02.007 & C.02.008
Raw Material Testing - C.02.009 & C.02.010
Manufacturing Control - C.02.011 & C.02.012
Quality Control Department - C.02.013, C.02.014 & C.02.015
Packaging Material Testing - C.02.016 & C.02.017
Finished Product Testing - C.02.018 & C.02.019
Records - C.02.020, C.02.021, C.02.022, C.02.023 & C.02.024
Samples - C.02.025 & C.02.026
Stability - C.02.027 & C.02.028
Sterile Products - C.02.029
CASE STUDY 6

http://www.pharmtech.com/early-development-gmps-drug-product-manufacturing-small-molecules-industry-perspective-part-iii?rel=canonical
Also
- Part VII GMPs for Small Molecule Drugs in Early Development Workshop Summary
- Early Development GMPs for Small-Molecule Specifications: An Industry Perspective (Part V)
- Early Development GMPs for Stability (Part IV)
- GMPs for Method Validation in Early Development: An Industry Perspective (Part II)
- GMPs for Small-Molecule Drugs in Early Development (Part I)
- GMPs for Small-Molecule Drugs in Early Development: Workshop Summary-Part VI
CASE STUDY 7
 http://www.niper.gov.in/tdc_2013.pdf
http://www.niper.gov.in/tdc_2013.pdf
CASE STUDY 8
Multi-kilo scale-up under GMP conditions
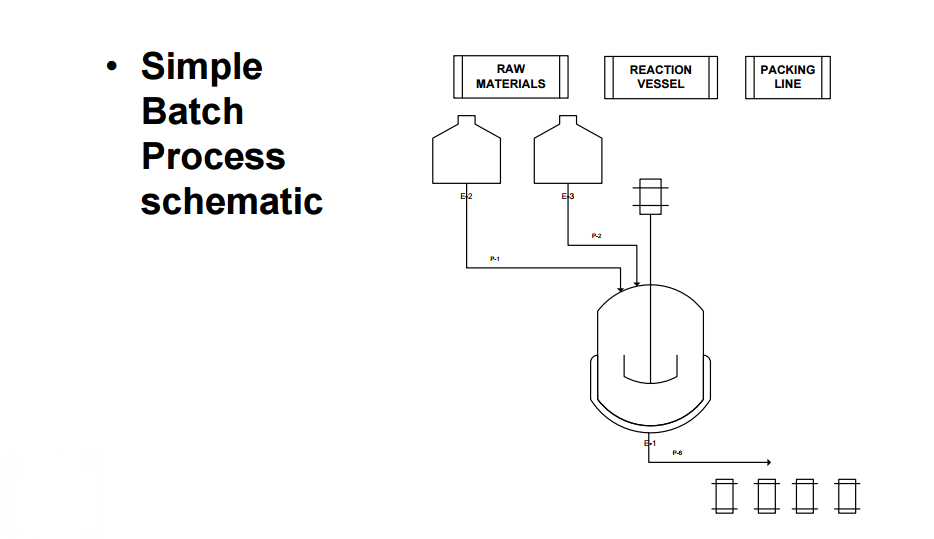
Examples of flow processes being used to produce exceptionally large amounts of material are becoming increasingly common as industrial researchers become more knowledgeable about the benefits of continuous reactions. The above examples from academic groups serve to illustrate that reactions optimized in small reactors processing tens to hundreds of mg hour−1 of material can be scaled up to several grams per hour. Projects in process chemistry are often time-sensitive, however, and production of multiple kg of material may be needed in a short amount of time. An example of how the efficient scaling of a flow reaction can save time and reduce waste is provided by a group of researchers at Eli Lilly in their kg synthesis of a key drug intermediate under GMP conditions . In batch, ketoamide 13 was condensed with NH4OAc and cyclized to form imidazole 14 at 100 °C in butanol on a 1 gram scale. However, side product formation became a significant problem on multiple runs at a 250 g scale. It was proposed that this was due to slow heat up times of the reactor with increasing scale, as lower temperatures seemed to favour increased degradation over productivecyclization. Upon switching to a 4.51 mL flow reactor, another optimization was carried out which identified methanol as a superior solvent that had been neglected in batch screening due to its low boiling point at atmospheric pressure. Scale-up to a 7.14 L reactor proceeded smoothly without the need for reoptimization, and running on this scale with a residence time of 90 minutes for a six-day continuous run provided 29.2 kg of product after recrystallization (approximately 207 g hour−1). The adoption of a flow protocol by a group of industrial researchers in a scale-up with time constraints demonstrates both the effectiveness and maturity of flow chemistry. While the given reaction was used to produce kilograms of material for a deadline, continuous operation without further optimization could produce over 1 metric tonne of product per year in a reactor that fits into a GC oven.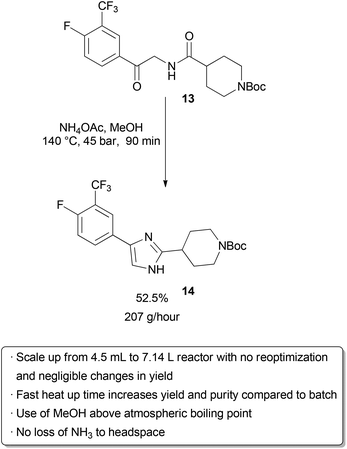 |
||
| Scheme 20 Kilogram-scale synthesis of an imidazole API precursor. | ||
SCALING UP FROM PILOT PLANTS
When scaling up, it is of utmost importance to consider all aspects of risk and futuristic expansion. The pilot plant is usually a costly apparatus and therefore the decision of building it is always a hard one. The function of a pilot plant is not just to prove that the laboratory experiments work, but;- To test technologies that are about to be implemented on industrial plants before establishment
- To evaluate performance specifications before the actual installation of industrial plant.
- Evaluation of reliability of mathematical models within real environment.
- Economic considerations for production involving process optimization and automated control systems.
GMP GENERAL PRACTISES
Facilities and Equipment Systems- Ø Cleaning and maintenance
- Ø Facility layout and air handling systems for prevention of cross-contamination (e.g. Penicillin, beta-lactams, steroids, hormones, cytotoxic, etc.)
- Ø Specifically designed areas for the manufacturing operations performed by the firm to prevent contamination or mix-ups.
- Ø General air handling systems
- Ø Control system for implementing changes in the building
- Ø Lighting, potable water, washing and toilet facilities, sewage and refuse disposal
- Ø Sanitation of the building, use of rodenticides, fungicides, insecticides, cleaning and sanitizing agents.
GMP FOR PLANT DESIGN
The application of GMP to plant design is primary to the establishment of such plants. Regulatory boards have precedence over these operations helping to establish a proper and functional system in plant design. Design Review l Conceptual drawings; From plant design drawings which are inspected and approved by cGMP regulatory bodies (such as Department of Petroleum Resources in Nigeria), approvals are issued depending on adherence to specifications such as muster points, proper spacing of fuel sources from combustion units and other more elaborate considerations. l Proposed plant layouts; A choice of location for plant and layout play an important role on environmental impact. Hence, environmental impact assessment is a major part of GMP. Industries must be located at least 100M from closest residential quarter (depending of materials processed in plant). l Flow diagrams for facility For optimization and efficiency purposes, flow diagrams for complete refinery process are important for review with intent to ensure they conform to GMP l Critical systems and areas Some areas in a plant may require extra safety precautions in operations. The cGMP makes provision for such special considerations with the creation of customized set of operational guidelines that ensure safety and wellness of staff and environment alike. cGMP EXAMPLE: FOOD PROCESSING PLANT Outlined below are the cGMP considerations in the establishment and handling of a food processing plant. Safety of Water 1. Process water is safe, if private supply should be tested at least annually. 2. Backflow prevention by an air gap or back flow prevention device. Sinks that are used to prepare food must have an air-gap. Food Contact Surface 1. Designed, maintained, and installed so that it is easy to clean and to withstand the use, environment, and cleaning compounds. 2. If cleaning is necessary to protect against microorganisms, food-contact surfaces shall be cleaned in this sequence: wash with detergent, rinse with clear water, and then use an approved sanitizer. The sanitizer used shall be approved for use on food-contact surfaces. UA three-compartment ware washing sink or other equivalent methods shall be used for this purpose. 3. Gloves shall be clean/sanitary. Outer garments suitable. Prevention of Cross-Contamination 1. Food handlers use good hygienic practices; hands shall be washed before starting work, after absence from work station, or when they become contamination (such as with eating or smoking). 2. Signs shall be posted in processing rooms and other appropriate areas directing employees that handle unprotected food, food-contact surfaces, food packaging materials to wash their hands prior to starting to work, after each absence from the work station, and whenever hands may become contaminated. 3. Plant design so that the potential for contamination of food, food-contact surfaces, or packaging materials is reduced to the extent possible. 4. Physical separation of raw and finished products. Hand Washing Sinks and Toilet Facilities 1. Hand washing sinks, properly equipped, shall be conveniently located to exposed food processing areas. Ware washing sinks shall not be used for this purpose. 2. Adequate supply of hot and cold water under pressure. 3. Toilet facilities; adequate and accessible, self-closing doors. 4. Sewage disposal system shall be installed and maintained according to State law. Protection from Adulteration (Food, Food Contact Surfaces, and Packaging Materials) 1. Food processing equipment designed to preclude contamination with lubricants, fuel, metal fragments, contaminated water, or other sources of contamination. 2. Food processed so that production methods to not contaminate the product. 3. Raw materials, works-in-process, filling, assembly, packaging, and storage and transportation conducted so that food is not contaminated. 4. Protection from drip and condensate overhead. 5. Ventilation adequate and air not blown on food or food-contact surfaces. 6. Lights adequately shielded. 7. Compressed air or gas mechanically introduced adequately filtered.Scope of services
- Engineering support
- Representation of the construction owner (equipment, construction: supervision of general contractors, GMP concept draft)
- Basic and detailed design
- Support during the implementation phase
- Clean room planning (incl. lab areas)
- Construction management
- Qualification
- Validation support

 Batch documentation and execution
Batch record documentation preparation. Manufacturing documentation is a basic requirement for all phases of clinical development. 21 CFR Parts 211.186 and 211.188 describe master production and batch production records, respectively (7). The stated purpose of the master production record is to "assure uniformity from batch to batch." Although the record assurance is important for a commercial validated manufacturing process, it does not necessarily apply to clinical-development batches. Material properties, manufacturing scale, and quality target product profile frequently change from batch to batch. Therefore, batch production records are the appropriate documentation for clinical trial supplies. Batch production records for Phase 1 materials should minimally include:
Batch documentation and execution
Batch record documentation preparation. Manufacturing documentation is a basic requirement for all phases of clinical development. 21 CFR Parts 211.186 and 211.188 describe master production and batch production records, respectively (7). The stated purpose of the master production record is to "assure uniformity from batch to batch." Although the record assurance is important for a commercial validated manufacturing process, it does not necessarily apply to clinical-development batches. Material properties, manufacturing scale, and quality target product profile frequently change from batch to batch. Therefore, batch production records are the appropriate documentation for clinical trial supplies. Batch production records for Phase 1 materials should minimally include:
- Name, strength, and description of the dosage form
- A complete list of active and inactive ingredients, including weight or measure per dosage unit and total weight or measure per unit
- Theoretical batch size (number of units)
- Manufacturing and control instructions.
 The two most popular and effective geometric scale-up methods are equal tip speed and equal power per volume. Equal tip speed results when the small-scale mixer speed is multiplied by the inverse geometric ratio of the impeller diameters to get the large-scale mixer speed:
N2 = N1(D1/D2)
Equal power per volume involves a similar calculation, except the geometry ratio is raised to the two-thirds power:
N2 = N1(D1/D2)(2/3)
This expression for power per volume only applies strictly for turbulent conditions, where the power number is constant, but is approximately correct for transition-flow mixing.
Avoid mix-ups
As we have seen, taking successive steps allows the development of alternative solutions to scale-up. Similar methods can be used to scale-down process problems for investigation in a pilot-plant or laboratory simulation. Here, too, non-geometric similarity often is a problem. Such scale-down calculations should help pinpoint appropriate operating speeds to test in the small-scale mixer.
In any scale-up or scale-down evaluation, some variables can be held constant while others must change. For example, even with geometric similarity, scale-up will result in less surface per volume because surface area increases as the length squared and volume increases as length cubed. Similarly, keeping blend time constant rarely is practical with any significant scale change. Larger tanks take longer to blend than smaller ones. Also, Reynolds number is expected to increase as size increases. In addition, standard operating speeds or available impeller sizes may necessitate a final adjustment to the scale-up calculations.
Rules for scale-up always have exceptions but understanding the effects of scale-up, especially non-geometric scale-up, can provide valuable guidance. Indeed, appreciation of the tradeoffs involved in non-geometric scale-up may be crucial for success with large-scale mixing processes.
The two most popular and effective geometric scale-up methods are equal tip speed and equal power per volume. Equal tip speed results when the small-scale mixer speed is multiplied by the inverse geometric ratio of the impeller diameters to get the large-scale mixer speed:
N2 = N1(D1/D2)
Equal power per volume involves a similar calculation, except the geometry ratio is raised to the two-thirds power:
N2 = N1(D1/D2)(2/3)
This expression for power per volume only applies strictly for turbulent conditions, where the power number is constant, but is approximately correct for transition-flow mixing.
Avoid mix-ups
As we have seen, taking successive steps allows the development of alternative solutions to scale-up. Similar methods can be used to scale-down process problems for investigation in a pilot-plant or laboratory simulation. Here, too, non-geometric similarity often is a problem. Such scale-down calculations should help pinpoint appropriate operating speeds to test in the small-scale mixer.
In any scale-up or scale-down evaluation, some variables can be held constant while others must change. For example, even with geometric similarity, scale-up will result in less surface per volume because surface area increases as the length squared and volume increases as length cubed. Similarly, keeping blend time constant rarely is practical with any significant scale change. Larger tanks take longer to blend than smaller ones. Also, Reynolds number is expected to increase as size increases. In addition, standard operating speeds or available impeller sizes may necessitate a final adjustment to the scale-up calculations.
Rules for scale-up always have exceptions but understanding the effects of scale-up, especially non-geometric scale-up, can provide valuable guidance. Indeed, appreciation of the tradeoffs involved in non-geometric scale-up may be crucial for success with large-scale mixing processes.
REFERENCES
1 https://docs.google.com/viewer?url=http%3A%2F%2Fwww.sunbio.com%2Fsub%2FSunbio%2520GMP%2520Capabilty.ppt 2 http://apic.cefic.org/pub/5gmpdev9911.pdf 3 http://www.pharmtech.com/early-development-gmps-drug-product-manufacturing-small-molecules-industry-perspective-part-iii?rel=canonical "ICH Q7a. Good Manufacturing Practice for Active Pharmaceutical Ingredients" (Draft 6, October 19th, 1999, section 19). "ICH Q6a. Specifications: test procedures and acceptance criteria for new drug substances and new drug products: chemical substances". "Good Manufacturing Practices for Active Pharmaceutical Ingredients" (EFPIA / CEFIC Guideline, August, 1996). "Quality Management System for Active Pharmaceutical Ingredients Manufacturers" (APIC/CEFIC May 1998). "Good Manufacturing Practices Guide for Bulk Pharmaceutical Excipients", The International Pharmaceutical Excipients Council (October 1995). "21 Code of Federal Regulations, parts 210 to 211", U.S. Food & Drug Administration. "Guide to inspection of Bulk Pharmaceutical Chemicals", U.S. Food & Drug Administration, (Revised Edition: May 1994). “Guidance for Industry. ANDAs: Impurities in Drug Substances”, U.S. Food and Drug Administration, CDER (June 1998). "Guideline on the Preparation of Investigational New Drug Products", U.S. Food & Drug Administration, CDER (March 1991). "EC Guides to GMP, Annex 13: Manufacture of Investigational Medicinal Products" (Revised Dec. 1996). "GMP Compliance during Development", David J. DeTora. Drug Information Journal, 33, 769-776, 1999. FDA Guidance documents on internet address: http://www.fda.gov/cder/guidance /index.htm EMEA Guidance documents on internet address: http://www.eudra.org. .................... DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
 amcrasto@gmail.com
amcrasto@gmail.com





















 COCK WILL TEACH YOU NMR
COCK WILL TEACH YOU NMR COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR