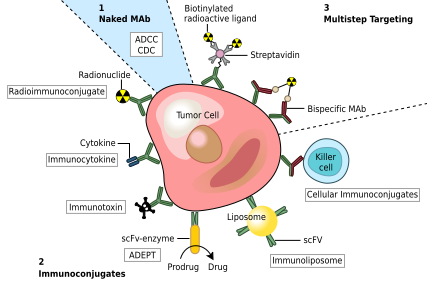
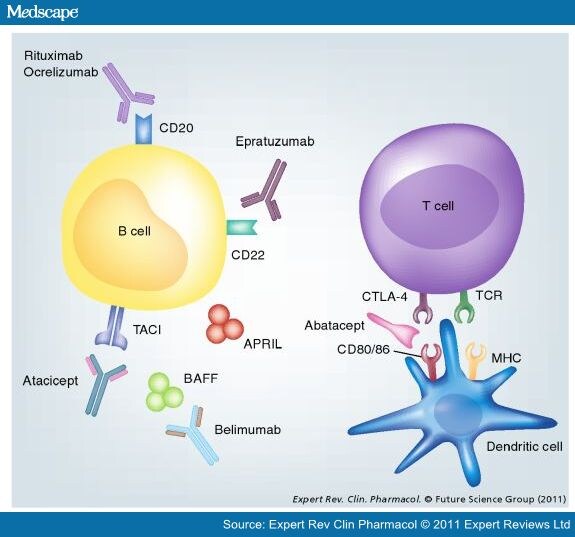
Novartis reports new Phase III data showing omalizumab significantly...
http://www.pharmalive.com/novartis-omalizumab-meets-phase-iii-endpoints
Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, helping millions with websites, 9 million hits on google, 2.5 lakh connections worldwide, P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.



 A new peptidomimetic inhibits prostate cancer’s growth by
preventing the androgen receptor from binding to its cofactor
A new peptidomimetic inhibits prostate cancer’s growth by
preventing the androgen receptor from binding to its cofactor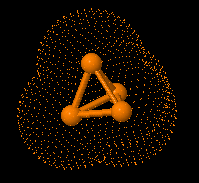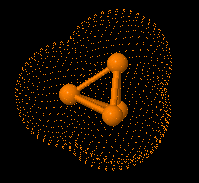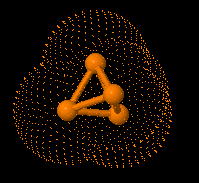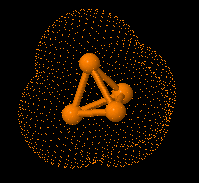
 Newly synthesized benzimidazole-type compounds work directly
in the gut rather than circulating in the blood to lower triglycerides
Newly synthesized benzimidazole-type compounds work directly
in the gut rather than circulating in the blood to lower triglycerides


 S. marianum
has been known and used to be recommended as an emetic. During the
Middle Ages the plant was probably cultivated in monasteries and used
for medicinal purposes: the roots, herb and leaves were recommended for
swelling and erysipelas (St. Hildegard from Bingen, 1098-1179) or for
the treatment of liver complaints (Lonicerus, John Gerard, Pietro Andrea
Mattioli, XVI-XVII centuries). From 1755 onwards, the specific use of
S. marianum fruit for the treatment of liver disease, disorders of the bile duct and spleen was documented. At present, the standardized extract (silymarin) obtained from the fruit of
S. marianum and containing as main constituents silybin,
silydianin and silychristin (Fig.1), is widely used in European medicine
in the treatment of liver disease. The main constituent silybin has
been subjected to several biochemical and pharmacological studies which
have demonstrated its interesting properties but also its poor
bioavailability. Complexation with soy phosphatidylcholine gives rise to
the lipophilic complex (US Patent 4, 764, 508) which
substantially improves the bioavailibility of silybin. This results in a
marked preventive action as observed in several models of liver
intoxication including those with a strong involvement of oxidative
stress. In this way, the silybin-phosphatidylcholine complex
SILIPHOS®, containing 33% of silybin, endowed with antioxidant activity
and, simultaneously, able to prevent cellular derangement by
stabilizing the cell membranes and restoring the normal ultrastructure
of the hepatocytes, plays a key role in the prevention of liver damage.http://www.swansonvitamins.com/health-library/products/siliphos.html
S. marianum
has been known and used to be recommended as an emetic. During the
Middle Ages the plant was probably cultivated in monasteries and used
for medicinal purposes: the roots, herb and leaves were recommended for
swelling and erysipelas (St. Hildegard from Bingen, 1098-1179) or for
the treatment of liver complaints (Lonicerus, John Gerard, Pietro Andrea
Mattioli, XVI-XVII centuries). From 1755 onwards, the specific use of
S. marianum fruit for the treatment of liver disease, disorders of the bile duct and spleen was documented. At present, the standardized extract (silymarin) obtained from the fruit of
S. marianum and containing as main constituents silybin,
silydianin and silychristin (Fig.1), is widely used in European medicine
in the treatment of liver disease. The main constituent silybin has
been subjected to several biochemical and pharmacological studies which
have demonstrated its interesting properties but also its poor
bioavailability. Complexation with soy phosphatidylcholine gives rise to
the lipophilic complex (US Patent 4, 764, 508) which
substantially improves the bioavailibility of silybin. This results in a
marked preventive action as observed in several models of liver
intoxication including those with a strong involvement of oxidative
stress. In this way, the silybin-phosphatidylcholine complex
SILIPHOS®, containing 33% of silybin, endowed with antioxidant activity
and, simultaneously, able to prevent cellular derangement by
stabilizing the cell membranes and restoring the normal ultrastructure
of the hepatocytes, plays a key role in the prevention of liver damage.http://www.swansonvitamins.com/health-library/products/siliphos.html
|

|
ruxolitinib
Incyte Drug Jakafi®ruxolitinib Improved Overall Survival in Phase III Trial of Patients with Myel. by Business Wirevia The Motley Fool Jun 16th 2013 220AM ... The phase III Controlled Myelofibrosis Study with Oral JAK Inhibitor-I (COMFORT-I) and COMFORT-II trials showed significant benefits by reducing spleen size, relieving debilitating symptoms, and improving overall survival  |







| |
Successful experiments on mice bode well for a future human contraceptive - if men can stomach the injections
Pet contraception is considered an important topic, given the four million unwanted dogs and cats that are thought to be put down every year in the US alone. Many vets routinely sterilise pets, but since surgery requires time and expertise scientists have been looking for cheaper, simpler alternatives. | |