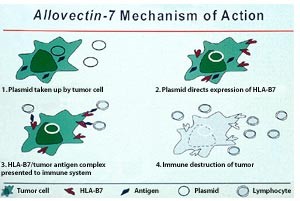
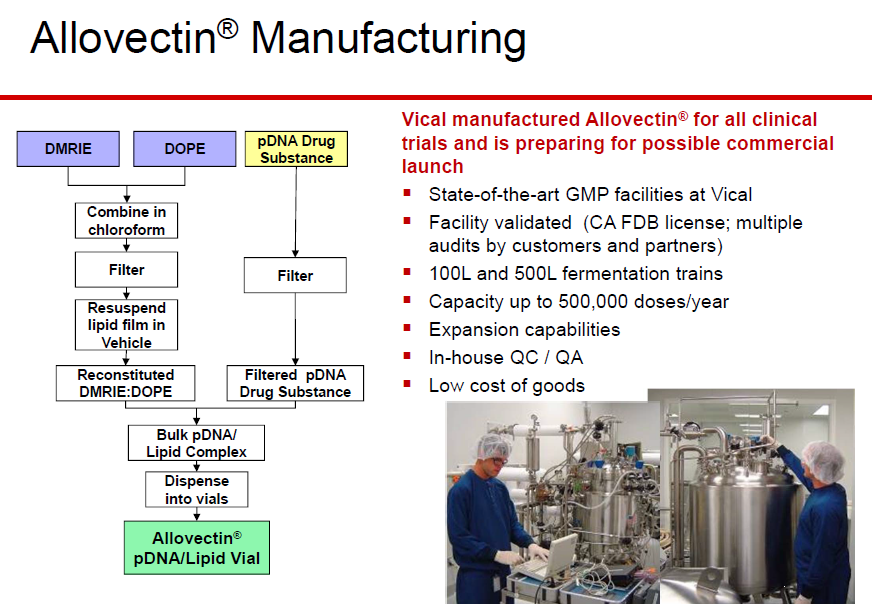
http://www.ama-assn.org/resources/doc/usan/eluxadoline.pdf
get the structure of eluxadoline here
name
Benzoic acid, 5-[[[(2S)-2-amino-3-[4-(aminocarbonyl)-2,6-dimethylphenyl]-1-
oxopropyl][(1S)-1-(5-phenyl-1H-imidazol-2-yl)ethyl]amino]methyl]-2-methoxy-
MOLECULAR FORMULA C32H35N5O5
MOLECULAR WEIGHT 569.7
TRADEMARK None as yet
SPONSOR Furiex Pharmaceuticals, Inc.
CODE DESIGNATIONS JNJ-27018966
CAS REGISTRY NUMBER 864821-90-9
WHO NUMBER 9749
read all at
http://www.pharmiweb.com/pressreleases/pressrel.asp?ROW_ID=76787#.UeUl4EHDBZg
need phase 2 data see here
In a phase 2 study of the mixed μ-opioid receptor agonist/δ-opioid receptor antagonist eluxadoline vs placebo in patients with IBS-D, patients given eluxadoline were significantly more likely to be clinical responders, based on a composite of improvement in abdominal pain and stool consistency. Further study of eluxadoline is warranted to assess its potential as a treatment for IBS-D. ClinicalTrials.gov number, NCT01130272
get the structure of eluxadoline here
name
Benzoic acid, 5-[[[(2S)-2-amino-3-[4-(aminocarbonyl)-2,6-dimethylphenyl]-1-
oxopropyl][(1S)-1-(5-phenyl-1H-imidazol-2-yl)ethyl]amino]methyl]-2-methoxy-
MOLECULAR FORMULA C32H35N5O5
MOLECULAR WEIGHT 569.7
TRADEMARK None as yet
SPONSOR Furiex Pharmaceuticals, Inc.
CODE DESIGNATIONS JNJ-27018966
CAS REGISTRY NUMBER 864821-90-9
WHO NUMBER 9749
Eluxadoline nonproprietary drug name - AMA
www.ama-assn.org/resources/doc/usan/eluxadoline.pdf
November 28, 2012. N11/133. STATEMENT ON A NONPROPRIETARY NAME ADOPTED BY THE USAN COUNCIL. USAN (ZZ-82). ELUXADOLINE.Furiex Pharmaceuticals Announces Completion of Patient Enrollment for Its Phase III Clinical Trials of Eluxadoline for IBS-d
Furiex Pharmaceuticals Inc.Posted on:15 Jul 13
Furiex Pharmaceuticals Inc. (NASDAQ: FURX) today announced completion of patient enrollment in the company’s two ongoing Phase III clinical trials studying eluxadoline for the treatment of diarrhea-predominant irritable bowel syndrome or IBS-d. Both studies met their target enrollments and Furiex expects to release top line results in the first quarter of 2014.
The two Phase III trials have the same overall design and efficacy endpoints but differ in overall duration. One study has a 52-week treatment period and the other a 30-week treatment period. Each study has three treatment arms placebo 75 mg eluxadoline twice a day and 100 mg eluxadoline twice a day with approximately 375 patients per arm and is designed to capture both the U.S. Food
The two Phase III trials have the same overall design and efficacy endpoints but differ in overall duration. One study has a 52-week treatment period and the other a 30-week treatment period. Each study has three treatment arms placebo 75 mg eluxadoline twice a day and 100 mg eluxadoline twice a day with approximately 375 patients per arm and is designed to capture both the U.S. Food
read all at
http://www.pharmiweb.com/pressreleases/pressrel.asp?ROW_ID=76787#.UeUl4EHDBZg
need phase 2 data see here
In a phase 2 study of the mixed μ-opioid receptor agonist/δ-opioid receptor antagonist eluxadoline vs placebo in patients with IBS-D, patients given eluxadoline were significantly more likely to be clinical responders, based on a composite of improvement in abdominal pain and stool consistency. Further study of eluxadoline is warranted to assess its potential as a treatment for IBS-D. ClinicalTrials.gov number, NCT01130272