'via Blog this'

Eddie Kehoe
Principal & Technical Director at Tcipatent Ltd
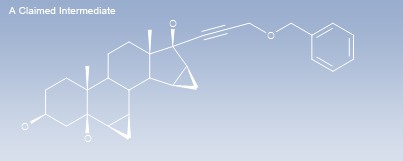
Hove, Brighton and Hove, United KingdomPharmaceuticalsThe Claimed Intermediate – a Structure Searchable Process Patent Database for Marketed Pharmaceutical Drugs (INNs).
Patent examining, searching, analysis and abstracting especially in the Chemical subject area.
Patent examining, searching, analysis and abstracting especially in the Chemical subject area.

The Claimed Intermediate is an online database
which covers Process Patents for Named Marketed Pharmaceutical Drugs – whether intermediates are claimed or not – for a low-cost subscription.
which covers Process Patents for Named Marketed Pharmaceutical Drugs – whether intermediates are claimed or not – for a low-cost subscription.
- Structure Searchable
- Includes INNs in at least one major Market
- Includes Drug Synthesis often buried in a Plethora of Patents
- Informs Pipeline decisions
- Provides targeted Patent data in a Visual form
- Informs Commercial Synthesis profitability

shared message from Eddie Kehoe
If anybody would like a trial of the database they could contact either myself eddie.kehoe@tcipatent.com, or my wife and fellow director, Pat Kehoe (pat.kehoe@tcipatent.com).
Here are temporary logons , please request trial
(deactivated automatically in five working days):
Link: Link: www.tcipatent.com/tcidb/
Structure Searchable Patent Database for Processes covering Named Marketed Pharmaceutical Drugs (INNs). The database is an ongoing Watching Service combined with a Backward Drug Service.
Link: Link: www.tcipatent.com/tcidb/
Structure Searchable Patent Database for Processes covering Named Marketed Pharmaceutical Drugs (INNs). The database is an ongoing Watching Service combined with a Backward Drug Service.
info@tcipatent.om
tcipatent.com
Office: +44 (0)1273 736080
43 Farm Road, Hove, BN3 1FD, United Kingdom
Office: +44 (0)1273 736080
43 Farm Road, Hove, BN3 1FD, United Kingdom
Eddie Kehoe:
eddie.kehoe@tcipatent.com
Mobile – 07425629637
Skype – eddieskihoe
eddie.kehoe@tcipatent.com
Mobile – 07425629637
Skype – eddieskihoe
TWITTER-TCIPATENT
Pat Kehoe:
pat.kehoe@tcipatent.com
Mobile – 07585295531
Skype – patkehoe170348
pat.kehoe@tcipatent.com
Mobile – 07585295531
Skype – patkehoe170348
Database Updates:
Recently Added Records
Recently Added Records
| Aliskiren | Ambrisentan |
| Asenapine | Atorvastatin |
| Bosentan | Cabazitaxel |
| Cefamandole | Dasatinib |
| Desogestrel | Dexmedetomidine |
| Docetaxel | Doripenem |
| Doxapram | Duloxetine |
| Etonogestrel | Etoricoxib |
| Etravirine | Fluvastatin |
| Gefitinib | Iodixanol |
| Iohexol | Iopamidol |
| Linagliptin | Mitiglinide |
| Montelukast | Moxonidine |
| Oseltamivir | Paclitaxel |
| Perampanel | Pitavastatin |
| Pravastatin | Praziquantel |
| Ritodrine | Rosuvastatin |
| Silodosin | Sitagliptin |
| Ticagrelor | Ulipristal |
| Zidovudine |
………..

photo
Coopers Cask - Pub in Hove BN3 1FB
Eddie is closeby