A vitamin (US /ˈvaɪtəmɪn/ or UK /ˈvɪtəmɪn/) is an organic compound required by an organism as a vital nutrient in limited amounts. An organic chemical compound (or related set of compounds) is called a vitamin when it cannot be synthesized in sufficient quantities by an organism, and must be obtained from the diet. Thus, the term is conditional both on the circumstances and on the particular organism. For example, ascorbic acid (vitamin C) is a vitamin for humans, but not for most other animals, and biotin (vitamin H) and vitamin D are required in the human diet only in certain circumstances.
Vitamin A - discovered in 1913
What it does:
Foods that have vitamin A:
Deficiency problems:
|  |
|
What it does:
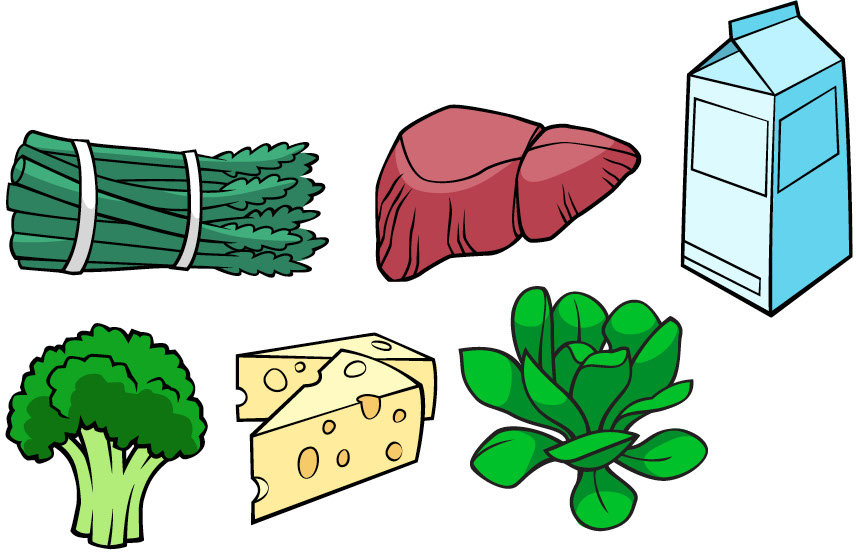
Foods that have vitamin D:
Deficiency problems:
|  |
|
What it does:
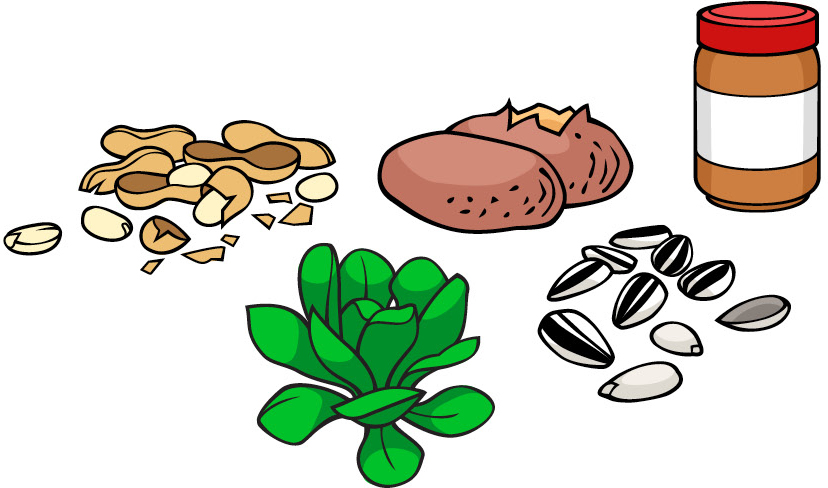
Foods that have vitamin E:
Deficiency problems:
|  |
Vitamin K - made by bacteria in our intestines
What it does:
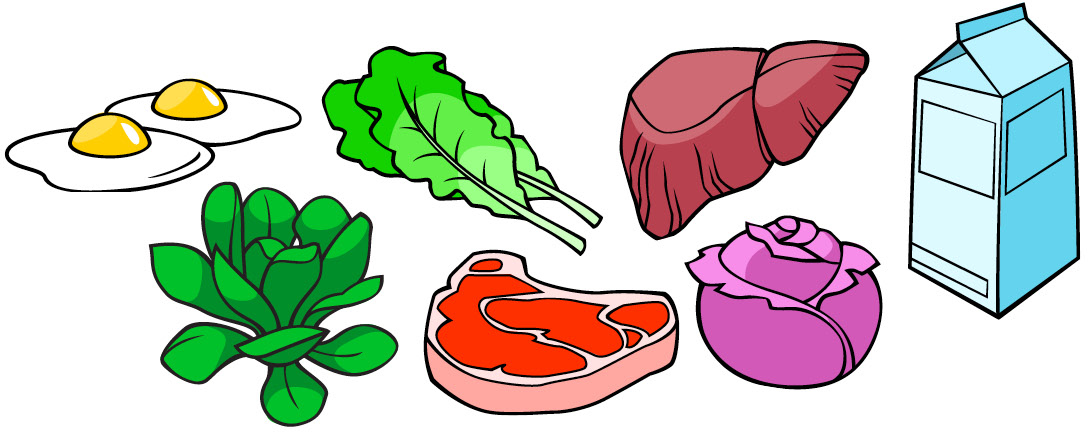
Foods that have vitamin K:
Deficiency problems:
|  |
By convention, the term vitamin includes neither other essential nutrients, such as dietary minerals, essential fatty acids, or essential amino acids (which are needed in larger amounts than vitamins) nor the large number of other nutrients that promote health but are otherwise required less often. Thirteen vitamins are universally recognized at present.
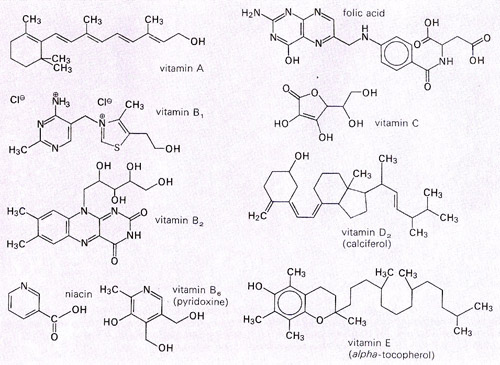
Vitamins are classified by their biological and chemical activity, not their structure. Thus, each "vitamin" refers to a number of vitamer compounds that all show the biological activity associated with a particular vitamin. Such a set of chemicals is grouped under an alphabetized vitamin "generic descriptor" title, such as "vitamin A", which includes the compounds retinal, retinol, and four known carotenoids. Vitamers by definition are convertible to the active form of the vitamin in the body, and are sometimes inter-convertible to one another, as well.
itamins have diverse biochemical functions. Some, such as vitamin D, have hormone-like functions as regulators of mineral metabolism, or regulators of cell and tissue growth and differentiation (such as some forms of vitamin A). Others function as antioxidants (e.g., vitamin E and sometimesvitamin C). The largest number of vitamins, the B complex vitamins, function as precursors for enzyme cofactors, that help enzymes in their work as catalysts in metabolism. In this role, vitamins may be tightly bound to enzymes as part of prosthetic groups: For example, biotin is part of enzymes involved in making fatty acids. They may also be less tightly bound to enzyme catalysts as coenzymes, detachable molecules that function to carry chemical groups or electrons between molecules. For example, folic acid may carry methyl, formyl, and methylene groups in the cell. Although these roles in assisting enzyme-substrate reactions are vitamins' best-known function, the other vitamin functions are equally important.

Until the mid-1930s, when the first commercial yeast-extract vitamin B complex and semi-synthetic vitamin C supplement tablets were sold, vitamins were obtained solely through food intake, and changes in diet (which, for example, could occur during a particular growing season) usually greatly altered the types and amounts of vitamins ingested. However, vitamins have been produced as commodity chemicals and made widely available as inexpensive semisynthetic and synthetic-source multivitamin dietary and food supplements and additives, since the middle of the 20th century.,,,,,,,

List of vitamins
Each vitamin is typically used in multiple reactions, and, therefore, most have multiple functions.
| Vitamin generic descriptor name | Vitamerchemical name(s) (list not complete) | Solubility | Recommended dietary allowances (male, age 19–70)[6] | Deficiency disease | Upper Intake Level (UL/day)[6] | Overdose disease | Food sources |
|---|---|---|---|---|---|---|---|
| Vitamin A | Retinol, retinal, and four carotenoids including beta carotene | Fat | 900 µg | Night-blindness,Hyperkeratosis, andKeratomalacia[7] | 3,000 µg | Hypervitaminosis A | Orange, ripe yellow fruits, leafy vegetables, carrots, pumpkin, squash, spinach, liver, soy milk, milk |
| Vitamin B1 | Thiamine | Water | 1.2 mg | Beriberi, Wernicke-Korsakoff syndrome | N/D[8] | Drowsiness or muscle relaxation with large doses.[9] | Pork, oatmeal, brown rice, vegetables, potatoes, liver, eggs |
| Vitamin B2 | Riboflavin | Water | 1.3 mg | Ariboflavinosis | N/D | Dairy products, bananas, popcorn, green beans, asparagus | |
| Vitamin B3 | Niacin, niacinamide | Water | 16.0 mg | Pellagra | 35.0 mg | Liver damage (doses > 2g/day)[10] and other problems | Meat, fish, eggs, many vegetables, mushrooms, tree nuts |
| Vitamin B5 | Pantothenic acid | Water | 5.0 mg[11] | Paresthesia | N/D | Diarrhea; possibly nausea and heartburn.[12] | Meat, broccoli, avocados |
| Vitamin B6 | Pyridoxine,pyridoxamine,pyridoxal | Water | 1.3–1.7 mg | Anemia[13] peripheral neuropathy. | 100 mg | Impairment ofproprioception, nerve damage (doses > 100 mg/day) | Meat, vegetables, tree nuts, bananas |
| Vitamin B7 | Biotin | Water | 30.0 µg | Dermatitis, enteritis | N/D | Raw egg yolk, liver, peanuts, certain vegetables | |
| Vitamin B9 | Folic acid, folinic acid | Water | 400 µg | Megaloblastic anemiaand Deficiency during pregnancy is associated with birth defects, such as neural tube defects | 1,000 µg | May mask symptoms of vitamin B12 deficiency;other effects. | Leafy vegetables, pasta, bread, cereal, liver |
| Vitamin B12 | Cyanocobalamin,hydroxycobalamin,methylcobalamin | Water | 2.4 µg | Megaloblastic anemia[14] | N/D | Acne-like rash [causality is not conclusively established]. | Meat and other animal products |
| Vitamin C | Ascorbic acid | Water | 90.0 mg | Scurvy | 2,000 mg | Vitamin C megadosage | Many fruits and vegetables, liver |
| Vitamin D | Cholecalciferol | Fat | 10 µg[15] | Rickets andOsteomalacia | 50 µg | Hypervitaminosis D | Fish, eggs, liver, mushrooms |
| Vitamin E | Tocopherols,tocotrienols | Fat | 15.0 mg | Deficiency is very rare; mild hemolytic anemiain newborn infants.[16] | 1,000 mg | Increased congestive heart failure seen in one large randomized study.[17] | Many fruits and vegetables, nuts and seeds |
| Vitamin K | phylloquinone,menaquinones | Fat | 120 µg | Bleeding diathesis | N/D | Increases coagulation in patients taking warfarin.[18] | Leafy green vegetables such as spinach, egg yolks, liver |