ALIROCUMAB « New Drug Approvals:
'via Blog this'
Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, helping millions with websites, 9 million hits on google, 2.5 lakh connections worldwide, P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
Thursday, 17 October 2013
Wednesday, 16 October 2013
En route to full implementation: driving the green chemistry agenda in the pharmaceutical industry
En route to full implementation: driving the green chemistry agenda in the pharmaceutical industry
Green Chem., 2013, Advance Article
DOI: 10.1039/C3GC41629A, Perspective
DOI: 10.1039/C3GC41629A, Perspective
Hans-Jurgen Federsel
Focus on the outlook for a green future in the pharmaceutical industry - close-up of historical drivers, blockers, challenges, and changes.
Focus on the outlook for a green future in the pharmaceutical industry - close-up of historical drivers, blockers, challenges, and changes.
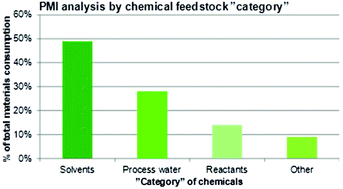
What is the relationship between the Green Chemistry initiative and the pharmaceutical industry? The intention is to shed some light on this issue by providing an historical overview spanning a period of about 20 years – from the start of the movement towards greener processes and manufacture in the early 1990s until today where greenness and sustainability are widely embraced throughout society. To understand and appreciate the approach to the green paradigm from a pharmaceutical business point of view, it is essential to paint the broader picture explaining the landscape in which this industry operates and its particular challenges. Looking at the special features that apply to chemical production of drug molecules for commercial use – in relative terms a low volume undertaking (from kg scale through to 10s or sometimes 100s of tons per annum) – the situation is vastly different compared to conventional bulk manufacture (for instance of commodity chemicals). After an initial lag phase, the drug industry has now caught up and is very eager to fully adopt green principles and to gather evidence on how it is performing. As an example, it is a well documented fact that more than half the mass constituting a process stream in the chemical manufacture of active pharmaceutical ingredients (APIs) stems from the solvent(s) utilized; 80–90% if water is included. In a multi-step synthesis on an average composed of 8–10 discrete chemical transformations which typically runs at a process mass intensity (PMI) factor of 100–200 kg kg−1API, about 50–100 kg can be referred purely to the contribution from solvents; hence, the potential for improvements is huge. Thus, leaving the historic priorities behind in favor of drivers for change such as external pressure, goodwill, legislation, and company policies is a good strategy to ensure a rapid movement into a greener future, albeit without ignoring the existence of blockers that mainly relate to insufficient scientific and technological capabilities. From a chemical process point of view, there are several reasons to have an optimistic view about the prospects of a flourishing green agenda going forward as shown in a number of recent case studies.
Tuesday, 15 October 2013
Monday, 14 October 2013
Sunday, 13 October 2013
VITAMIN A
................
Vitamin a from mallik813

Vitamin A is a group of nutritionally unsaturated hydrocarbons, which include retinol, retinal, retinoic acid, and several provitamin A carotenoids, among which beta-carotene is the most important. Vitamin A has multiple functions, it is important for growth and development, for the maintenance of the immune system and good vision. Vitamin A is needed by the retina of the eye in the form of retinal, which combines with protein opsin to form rhodopsin the light-absorbing molecule, that is necessary for both low-light (scotopic vision) and color vision. Vitamin A also functions in a very different role as an irreversibly oxidized form ofretinol known as retinoic acid, which is an important hormone-like growth factor for epithelial and other cells.
In foods of animal origin, the major form of vitamin A is an ester, primarily retinyl palmitate, which is converted to retinol (chemically an alcohol) in the small intestine. The retinol form functions as a storage form of the vitamin, and can be converted to and from its visually active aldehyde form, retinal. The associated acid (retinoic acid), a metabolite that can be irreversibly synthesized from vitamin A, has only partial vitamin A activity, and does not function in the retina for the visual cycle. Retinoic acid is used for growth and cellular differentiation.
All forms of vitamin A have a beta-ionone ring to which an isoprenoid chain is attached, called a retinyl group. Both structural features are essential for vitamin activity. The orange pigment of carrots – beta-carotene – can be represented as two connected retinyl groups, which are used in the body to contribute to vitamin A levels. Alpha-carotene and gamma-carotene also have a single retinyl group, which give them some vitamin activity. None of the other carotenes have vitamin activity. The carotenoid beta-cryptoxanthin possesses an ionone group and has vitamin activity in humans.
Vitamin A can be found in two principal forms in foods:
- Retinol, the form of vitamin A absorbed when eating animal food sources, is a yellow, fat-soluble substance. Since the pure alcohol form is unstable, the vitamin is found in tissues in a form of retinyl ester. It is also commercially produced and administered as esters such as retinyl acetate or palmitate.
- The carotenes alpha-carotene, beta-carotene, gamma-carotene; and the xanthophyll beta-cryptoxanthin (all of which contain beta-ionone rings), but no other carotenoids, function as provitamin A in herbivores and omnivore animals, which possess the enzyme (15-15'-dioxygenase) which cleaves beta-carotene in the intestinal mucosa and converts it to retinol.[9] In general, carnivores are poor converters of ionine-containing carotenoids, and pure carnivores such as cats and ferrets lack 15-15'-dioxygenase and cannot convert any carotenoids to retinal (resulting in none of the carotenoids being forms of vitamin A for these species).
History
The discovery of vitamin A may have stemmed from research dating back to 1816, when physiologist Magendie observed that dogs deprived of nutrition developed corneal ulcers and had high mortality rate. In 1912, Frederick Gowland Hopkins demonstrated that unknown "accessory factors" found in milk, other than carbohydrates, proteins, and fats were necessary for growth in rats. Hopkins received a Nobel Prize for this discovery in 1929. By 1917, one of these substances was independently discovered by Elmer McCollum at the University of Wisconsin–Madison, and Lafayette Mendel and Thomas Burr Osborne at Yale University who studied the role of fats in the diet. The "accessory factors" were termed "fat soluble" in 1918 and later "vitamin A" in 1920. In 1919, Harry Steenbock (University of Wisconsin) proposed a relationship between yellow plant pigments (beta-carotene) and vitamin A. In 1931, a Swiss Chemist Paul Karrer described the chemical structure of vitamin A.[13] Vitamin A was first synthesized in 1947 by two Dutch chemists; David Adriaan van Dorp and Jozef Ferdinand Arens.
Equivalencies of retinoids and carotenoids (IU
As some carotenoids can be converted into vitamin A, attempts have been made to determine how much of them in the diet is equivalent to a particular amount of retinol, so that comparisons can be made of the benefit of different foods. The situation can be confusing because the accepted equivalences have changed. For many years, a system of equivalencies in which an international unit (IU) was equal to 0.3 μg of retinol, 0.6 μg of β-carotene, or 1.2 μg of other provitamin-A carotenoids was used. Later, a unit called retinol equivalent (RE) was introduced. Prior to 2001, one RE corresponded to 1 μg retinol, 2 μg β-carotene dissolved in oil (it is only partly dissolved in most supplement pills, due to very poor solubility in any medium), 6 μg β-carotene in normal food (because it is not absorbed as well as when in oils), and 12 μg of either α-carotene, γ-carotene, or β-cryptoxanthin in food.
Newer research has shown that the absorption of provitamin-A carotenoids is only half as much as previously thought. As a result, in 2001 the US Institute of Medicinerecommended a new unit, the retinol activity equivalent (RAE). Each μg RAE corresponds to 1 μg retinol, 2 μg of β-carotene in oil, 12 μg of "dietary" beta-carotene, or 24 μg of the three other dietary provitamin-A carotenoids.
| Substance and its chemical environment | Micrograms of retinol equivalent per microgram of the substance |
|---|---|
| retinol | 1 |
| beta-carotene, dissolved in oil | 1/2 |
| beta-carotene, common dietary | 1/12 |
| alpha-carotene, common dietary | 1/24 |
| gamma-carotene, common dietary | 1/24 |
| beta-cryptoxanthin, common dietary | 1/24 |
Because the conversion of retinol from provitamin carotenoids by the human body is actively regulated by the amount of retinol available to the body, the conversions apply strictly only for vitamin A-deficient humans. The absorption of provitamins depends greatly on the amount of lipids ingested with the provitamin; lipids increase the uptake of the provitamin.
The conclusion that can be drawn from the newer research is that fruits and vegetables are not as useful for obtaining vitamin A as was thought; in other words, the IUs that these foods were reported to contain were worth much less than the same number of IUs of fat-dissolved oils and (to some extent) supplements. This is important forvegetarians, as night blindness is prevalent in countries where little meat or vitamin A-fortified foods are available.
A sample vegan diet for one day that provides sufficient vitamin A has been published by the Food and Nutrition Board (page 120). On the other hand, reference values for retinol or its equivalents, provided by the National Academy of Sciences, have decreased. The RDA (for men) of 1968 was 5000 IU (1500 μg retinol). In 1974, the RDA was set to 1000 RE (1000 μg retinol), whereas now the Dietary Reference Intake is 900 RAE (900 μg or 3000 IU retinol). This is equivalent to 1800 μg of β-carotene supplement (3000 IU) or 10800 μg of β-carotene in food (18000 IU).
Recommended daily intake
Vitamin A
Dietary Reference Intake:
Dietary Reference Intake:
| Life stage group | RDA
Adequate intakes (AI*)
μg/day | Upper limit
μg/day
|
|---|---|---|
| Infants
0–6 months
7–12 months | 400* 500* | 600 600 |
| Children
1–3 years
4–8 years | 300 400 | 600 900 |
| Males
9–13 years
14–18 years 19 – >70 years | 600 900 900 | 1700 2800 3000 |
| Females
9–13 years
14–18 years 19 – >70 years | 600 700 700 | 1700 2800 3000 |
| Pregnancy
<19 years
19 – >50 years | 750 770 | 2800 3000 |
| Lactation
<19 years
19 – >50 years | 1200 1300 | 2800 3000 |
(The limit is for synthetic and natural retinol ester forms of vitamin A. Carotene forms from dietary sources are not toxic.)
According to the Institute of Medicine of the National Academies, "RDAs are set to meet the needs of almost all (97 to 98%) individuals in a group. For healthy breastfed infants, the AI is the mean intake. The AI for other life stage and gender groups is believed to cover the needs of all individuals in the group, but lack of data prevents being able to specify with confidence the percentage of individuals covered by this intake."
Sources
Vitamin A is found naturally in many foods:
- cod liver oil (30000 μg)
- liver (turkey) (8058 μg)
- liver (beef, pork, fish) (6500 μg 722%)
- liver (chicken) (3296 μg)
- dandelion greens (5588 IU 112%)
- carrot (835 μg 93%)
- broccoli leaf (800 μg 89%) – According to USDA database broccoli florets have much less.
- sweet potato (709 μg 79%)
- butter (684 μg 76%)
- kale (681 μg 76%)
- spinach (469 μg 52%)
- pumpkin (400 μg 41%)
- collard greens (333 μg 37%)
- Cheddar cheese (265 μg 29%)
- cantaloupe melon (169 μg 19%)
- egg (140 μg 16%)
- apricot (96 μg 11%)
- papaya (55 μg 6%)
- mango (38 μg 4%)
- pea (38 μg 4%)
- broccoli (31 μg 3%)
- milk (28 μg 3%)
- tomatoes
- Seaweed
Note: data taken from USDA database bracketed values are retinol activity equivalences (RAEs) and percentage of the adult male RDA, per 100 grams of the foodstuff (average).
Conversion of carotene to retinol varies from person to person and bioavailability of carotene in food varies.

Subscribe to:
Comments (Atom)

