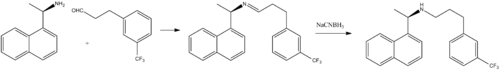
Popular drugs scheduled to lose U.S. market protection between 2013 and 2018:reader keep watching this post , I will keep updating with new info
Drugs worth an estimated $103 billion lost their patents between 2009 and 2012 with a further $29 million loss expected in 2013.(Source: DTTL Global Life Sciences and Health Care Industry Group analysis of Global Generic, Cygnus)
Some large pharmaceutical companies like Bristol Myers, Pfizer, Eli Lilly and Merck have prepared by buying smaller biotech companies that offer interesting alternatives to their drug pipelines, suggesting a refocus on recruitment in these developing areas.
Many are looking to replace their blockbuster money-spinners with smaller, more targeted treatments. A strong skills requirement for these new inventions is a positive move for some sectors.
A swift move in to emerging markets like Brazil, India and China and alternative business models means a more dynamic global recruitment drive and a greater effort to match skills, new locations and business management as quickly as possible.
Niche products – including so-called orphan drugs – are attractive but leave these larger companies looking at a loss if they can’t convince their local regulatory body that a branded version is more commercially viable than the generic alternative.
So is it the generic drug development projects that will see the biggest impact on Life Science recruitment in the next 18 months? Or will it be established ‘big pharma’ companies moving in to emerging markets?
Some large pharmaceutical companies like Bristol Myers, Pfizer, Eli Lilly and Merck have prepared by buying smaller biotech companies that offer interesting alternatives to their drug pipelines, suggesting a refocus on recruitment in these developing areas.
Many are looking to replace their blockbuster money-spinners with smaller, more targeted treatments. A strong skills requirement for these new inventions is a positive move for some sectors.
A swift move in to emerging markets like Brazil, India and China and alternative business models means a more dynamic global recruitment drive and a greater effort to match skills, new locations and business management as quickly as possible.
Niche products – including so-called orphan drugs – are attractive but leave these larger companies looking at a loss if they can’t convince their local regulatory body that a branded version is more commercially viable than the generic alternative.
So is it the generic drug development projects that will see the biggest impact on Life Science recruitment in the next 18 months? Or will it be established ‘big pharma’ companies moving in to emerging markets?
The global pharmaceutical market is witnessing a change as many patents of key blockbuster drugs have expired in last few years. The patent expiration of blockbuster drug fuel the growth of global generic drug pharmaceutical market. Many branded pharmaceutical companies drugs expired in last few years like Lipitor, Cozaar, Concerta,Crestor, Levaquin and Zyprexa. Market leaders such as Teva, Sandoz and Mylan are increasingly focused on generics, as this segment provides a competitive edge and presents huge profit margins. Patent expiration opens the door for generic versions which make the healthcare available to the consumers at cheaper costs as compared to the patented drugs.
According to IBISWorld, the top five best-sellers set to lose patent protection in each of the yrs 2011 and 2012 were
| Patent Expiring in 2011 | Condition | Company | 2010 U.S. Sales |
| Lipitor | cholesterol | Pfizer | $5,329,000,000 |
| Zyprexa | antipsychotic | Eli Lily | $2,496,000,000 |
| Levaquin | antibiotics | Johnson & Johnson | $1,312,000,000 |
| Concerta | ADHD/ADD | Johnson & Johnson | $929,000,000 |
| Protonix | antacid | Pfizer | $690,000,000 |
| Patent Expiring in 2012 | Condition | Company | 2010 U.S. Sales |
| Plavix | anti-platelet | Bristol-Myers Squibb / Sanofi-Aventis | $6,154,000,000 |
| Seroquel | antipsychotic | AstraZeneca | $3,747,000,000 |
| Singulair | asthma | Merck | $3,224,000,000 |
| Actos | type 2 diabetes | Takeda | $3,351,000,000 |
| Enbrel | arthritis | Amgen | $3,304,000,000 |

YEAR 2013



Ac-Tyr-Thr-Ser-Leu-Ile-His-Ser-Leu-Ile-Glu-Glu-Ser-Gln-Asn-Gln-Gln-Glu-Lys-Asn-Glu-Gln-Glu-Leu-Leu-Glu-Leu-Asp-Lys-Trp-Ala-Ser-Leu-Trp-Asn-Trp-Phe-NH2
enfuvirtide, fuzeon
Enfuvirtide (INN) is an HIV fusion inhibitor, the first of a novel class of antiretroviral drugs used in combination therapy for the treatment of HIV-1 infection. It is marketed under the trade name Fuzeon (Roche).
Enfuvirtide therapy costs an estimated US$25,000 per year in the United States. Its cost and inconvenient dosing regimen are factors behind its use as a reserve, for "salvage" therapy in patients with multi-drug resistant HIV.
USD 43 IN 2008

Epogen
Epoetin alfa (rINN) /ɛˈpoʊ.ɨtɨn/ is human erythropoietin produced in cell culture using recombinant DNA technology. Authorised by the European Medicines Agency on 28 August 2007, it stimulates erythropoiesis (increases red blood cell levels) and is used to treat anemia, commonly associated with chronic renal failure and cancer chemotherapy. Epoetin is marketed under the trade names Procrit and Epogen. Its annual cost to U.S. patients was $8,447 per patient per year in 2009
Amgen being the most hard hit of all the companies. Its blockbuster drug for anemia, Epogen, which had a sale of about $2.5 billion (12,500 crore) in 2008, will lose its patent in 2013.

Ac-Tyr-Thr-Ser-Leu-Ile-His-Ser-Leu-Ile-Glu-Glu-Ser-Gln-Asn-Gln-Gln-Glu-Lys-Asn-Glu-Gln-Glu-Leu-Leu-Glu-Leu-Asp-Lys-Trp-Ala-Ser-Leu-Trp-Asn-Trp-Phe-NH2
enfuvirtide, fuzeon
Enfuvirtide (INN) is an HIV fusion inhibitor, the first of a novel class of antiretroviral drugs used in combination therapy for the treatment of HIV-1 infection. It is marketed under the trade name Fuzeon (Roche).
Enfuvirtide therapy costs an estimated US$25,000 per year in the United States. Its cost and inconvenient dosing regimen are factors behind its use as a reserve, for "salvage" therapy in patients with multi-drug resistant HIV.
USD 43 IN 2008

Oxycontin (oxycodone HCI), an opioid for pain management, earned $2.8 billion in sales in 2011 for its U.S. manufacturer, Purdue Pharma LP.

AcipHex (rabeprazole sodium), for heartburn associated with acid reflux disease, manufacturer: Eisai Inc. and Janssen Pharmaceuticals, Inc.
Zometa (zoledronic acid) injection is a treatment for hypercalcemia of malignancy caused by high calcium blood levels due to cancer; $1.5 billion in global sales in 2010. Manufactured by Novartis.

Xeloda (capecitabine) is an oral chemotherapy treatment for metastatic colorectal and breast cancer manufactured by Genentech/Roche. U.S. sales were growing by double digits annually to reach $647.6 million in 2011.
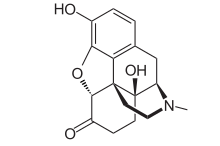
Oplana ER (oxymorphone HCI), an opioid agonist approved for management of moderate to severe pain, manufactured by Endo Pharmaceuticals Inc. Sales in 2011 were $384.7 million.

Asacol (mesalamine), for the treatment of ulcerative colitis symptoms, earned $800 million in worldwide sales in 2010. Manufacturer: Warner Chilcott

Another blockbuster drug Neupogen will lose its patent in 2013
Filgrastim is a granulocyte colony-stimulating factor (G-CSF) analog used to stimulate the proliferation and differentiation ofgranulocytes. It is produced by recombinant DNA technology. The gene for human granulocyte colony-stimulating factor is inserted into the genetic material of Escherichia coli. The G-CSF then produced by E. coli is different from G-CSF naturally made in humans.
Filgrastim is marketed under several brand names, including Neupogen (Amgen), Imumax (Abbott Laboratories), Grafeel (Dr. Reddy's Laboratories), Neukine (Intas Biopharmaceuticals), Emgrast (Emcure Pharmaceuticals), Religrast (Reliance Life Sciences), Zarzio (Sandoz), Nufil (Biocon) and others.
Apricus Biosciences is currently developing and testing a product under the brand name Nupen which can deliver filgrastim through the skin to improve post-chemotherapy recovery of neutrophil counts.
.................................................................................................
VANOS .................................................................................................
(fluocinonide)
Medicis
Dermatoses $98.6 million December 2013

Join me-Do not miss me. email me if you like me
http://newdrugapprovals.wordpress.com/patent-expiry/
YEAR 2014



TEMOZOLOMIDE
Temodar, temozolomide, dollar 228 million in 2008
YEAR 2014

Micardis (telmisartan)
Boehringer Ingelheim's angiotensin II receptor antagonist telmisartan is approved to treat hypertension and reduce the risk of cardiovascular events. With net sales of $17 billion reported in 2008, the firm will be looking to protect and extend the patent for this first drug in its class to reduce the risk of heart attack and stroke. Even with a black box warning advising against use during pregnancy, there is huge interest in the potential of the product; over 90 clinical trials have been or are being conducted to investigate its use in various patient groups, drug combinations and for other disease states.

Hectorol (doxercalciferol)
Genzyme's doxercalciferol, used to maintain consistent serum concentrations of vitamin D hormone, is approved for secondary hyperparathyroidism, and was acquired when the firm bought Bone Care International to gain products for kidney disease. Trials testing the drug for osteoporosis and prostate cancer are also underway. Sales, posted at $128 million in 2008, should be enhanced by the introduction of a vial formulation to replace a more awkward glass ampoule as well as a 1mcg capsule. Genzyme is actively defending its patent protections; in July 2009 the company sued the Sandoz unit of Novartis for alleged infringement.

TEMOZOLOMIDE
Temodar, temozolomide, dollar 228 million in 2008
| TEMODAR® (Pediatric) | TEMOZOLOMIDE | exp2014-02 | SCHERING |

Nexium (esomeprazole) for the treatment of gastroesophageal reflux disease (GERD) is manufactured by AstraZeneca. Global sales in 2010 were $4.9 billion.
AstraZeneca's esomeprazole, indicated for acid reflux and erosive esophagitis, has held its own in sales among other branded products such as Prevacid, and remains one of the world’s largest selling drugs. This may not last. The advent of generics for the same indications, now accounting for 40% of marketed products, have cut into Nexium’s market share for the "purple pill" – which last year amounted to $5.9 billion. The proton pump inhibitor's patent history is somewhat controversial, generating some charges that this enantiomer of omeprazole provides little advantage or change but is an attempt to "evergreen" the product. In the end, though, it's an attractive market, and AstraZeneca has already brought the Indian firm Lupin to court, alleging patent infringement. Barring successful patent challenges from other firms, the first generic on the market will be Ranbaxy, licensed in 2008 by AstraZeneca to begin distribution six months before patent expiration. Finally, esomepazole carries two patents not expiring until 2018, and the trade press has wondered if these are an ace up AstraZeneca’s patent lawyers' sleeves.
AstraZeneca's esomeprazole, indicated for acid reflux and erosive esophagitis, has held its own in sales among other branded products such as Prevacid, and remains one of the world’s largest selling drugs. This may not last. The advent of generics for the same indications, now accounting for 40% of marketed products, have cut into Nexium’s market share for the "purple pill" – which last year amounted to $5.9 billion. The proton pump inhibitor's patent history is somewhat controversial, generating some charges that this enantiomer of omeprazole provides little advantage or change but is an attempt to "evergreen" the product. In the end, though, it's an attractive market, and AstraZeneca has already brought the Indian firm Lupin to court, alleging patent infringement. Barring successful patent challenges from other firms, the first generic on the market will be Ranbaxy, licensed in 2008 by AstraZeneca to begin distribution six months before patent expiration. Finally, esomepazole carries two patents not expiring until 2018, and the trade press has wondered if these are an ace up AstraZeneca’s patent lawyers' sleeves.

Cymbalta (duloxitine HCI), manufactured by Eli Lilly, is for the treatment of depression, generalized anxiety disorder, diabetic nerve pain, fibromylagia and chronic musculoskeletal pain. Global sales exceeded $4 billion in 2011.

Celebrex (celecoxib) is for the treatment of osteoarthritis and rheumatoid arthritis symptoms and management of acute pain in adults. Pfizer, its manufacturer, reported sales of $2.5 billion in 2011.
Marketed by Pfizer, celecoxib, a COX II inhibitor, is indicated for arthritis pain, other acute pain, and primary dysmenorrhea. It is also marketed as Onsenal for familial adenomatous polyps. Available already as a generic in India and the Philippines, the drug was originally developed by Searle and co-promoted by Pfizer and Monsanto (whose research division was eventually acquired by Pfizer). Searle had already prevailed in a patent suit with The University of Rochester, and with Vioxx's market withdrawal in 2004, Celebrex enjoyed a large market share, even with a clinical trial underway investigating the cardiovascular risks now described in a black box warning. Pfizer expected patent exclusivity until 2015, but a Federal Circuit court, siding with Teva, invalidated the patent in question and resulted in the loss of approximately 18 months of patent protection for Pfizer. Watch for generics on May 30, 2014, when the pediatric exclusivity expires.

RIZATRIPTAN
Maxalt, rizatriptan, USdollar 224 in 2008
Marketed by Pfizer, celecoxib, a COX II inhibitor, is indicated for arthritis pain, other acute pain, and primary dysmenorrhea. It is also marketed as Onsenal for familial adenomatous polyps. Available already as a generic in India and the Philippines, the drug was originally developed by Searle and co-promoted by Pfizer and Monsanto (whose research division was eventually acquired by Pfizer). Searle had already prevailed in a patent suit with The University of Rochester, and with Vioxx's market withdrawal in 2004, Celebrex enjoyed a large market share, even with a clinical trial underway investigating the cardiovascular risks now described in a black box warning. Pfizer expected patent exclusivity until 2015, but a Federal Circuit court, siding with Teva, invalidated the patent in question and resulted in the loss of approximately 18 months of patent protection for Pfizer. Watch for generics on May 30, 2014, when the pediatric exclusivity expires.

RIZATRIPTAN
Maxalt, rizatriptan, USdollar 224 in 2008
| Maxalt® | rizatriptan | exp2014-02 | Migraines | MERCK |

BUDESONIDE

formoterol fumarate dihydrate
Symbicort (budesonide/formoterol fumarate dihydrate) for asthma and COPD is manufactured by AstraZeneca. Sales of Symbicort were $3.1 billion in 2011.
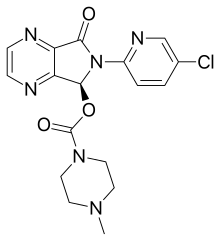
Lunesta (Eszopiclone), a treatment for insomnia, is manufactured by Sunovion Pharmaceuticals Inc., a subsidiary of Dainippon Sumitomo Pharma Co. Ltd. Global sales in 2010 were $631 million.

Restasis (cyclosporine ophthalmic emulsion) for increasing tear production in patients with chronic dry eyes, is manufactured by Allergan. In 2010, worldwide sales reached $621 million.

Evista (raloxifene HCI), manufactured by Eli Lilly, is for treating and preventing osteoporosis. Evista earned $1.3 billion in global sales in 2010.
H-D-Phe-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr-ol,
xCH3COOH where x = 1.4 to 2.5
Sandostatin LAR (octreotide acetate for injectable suspension) by Novartis, is for the treatment of acromegaly syndrome, a hormonal disorder, and severe diarrhea and flushing associated with metastatic cancers. Global sales were $1.3 billion in 2010.
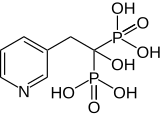
Actonel (risedronate) is for the prevention and treatment of osteoporosis. In 2010, worldwide sales were $1.6 billion. Actonel is manufactured by Warner Chilcott.

MOXIFLOXACIN
Avelox, moxifloxacin. us dollar 500 on 2008
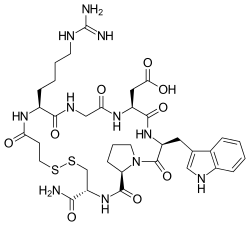
or

Eptifibatide, CAS# 188627-80-7, is an antiplatelet agent. Also offered from LGM Pharma as a TEVA API (TAPI) product for compounding purposes, eptifibatide is also known by the brand name Integrilin, which is marketed by Merck. With Merck’s patent for Integrilin due to expire on November 11, 2014, and an additional patent revision due to expire on May 5, 2015, this powerful drug is set to be a generic blockbuster. -
....................................................................................................
RAPAMUNE Oral Tab‡
(sirolimus)
Wyeth/Pfizer
Prevention of Organ Transplant Rejection
$231 million January 2014

(sirolimus)
Wyeth/Pfizer
Prevention of Organ Transplant Rejection
$231 million January 2014

Join me-Do not miss me
YEAR 2015

BARACLUDE® is the tradename for entecavir
Baraclude - After Plavix, Baraclude is the next significant challenge that BMS will have to face immediately. It is used in the treatment of chronic hepatitis B infection and fetched almost $1.4 billion in revenues for the firm in 2012. In February 2013, the composition of matter patent covering this drug was invalidated by the U.S. district court for the district of Delaware. Originally, the patent was scheduled to expire in 2015, but now the drug may face generic competition in the U.S. as early as in 2013. The firm will continue to sell in other parts of the world without competition from generic drugs until 2016.

Abilify (aripiprazole) by Bristol-Myers Squibb is an anti-psychotic used to treat the symptoms of schizophrenia and bipolar disorder. In 2010, global sales reached $4.6 billion.
Included here because of its original patent expiration in 2014, Bristol-Myers has won a pediatric exclusivity extension for aripiprazole until April, 2015...unless Teva prevails in its patent challenge initiated in 2007. Judging by the product's growth rate, watch for more patent challenges – in 2009, the product yielded $606 million – a 31% jump over the previous year. Indicated for bipolar disorders, schizophrenia, autism and as an add-on to an antidepressant, aripiprazole was recently approved for treatment of children over 10 for bipolar disorder, and the product carries the expected atypical antipsychotic black box warnings for suicidal thoughts in children and young adults.
The antipsychotic and anti-depressant drug is the next bestselling drug after Plavix. The drug was developed by Otsuka in Japan, and it markets the drug in partnership with BMS in the U.S and Europe. The drug brought in $2.8 billion in revenues for BMS in 2012. However, BMS’s rights to commercialize the drug in EU and U.S. will end in 2014 and 2015 respectively. Simultaneously, Otsuka’s U.S. patent for the drug expires in 2014, and the drug will face competition from generic products soon.
Included here because of its original patent expiration in 2014, Bristol-Myers has won a pediatric exclusivity extension for aripiprazole until April, 2015...unless Teva prevails in its patent challenge initiated in 2007. Judging by the product's growth rate, watch for more patent challenges – in 2009, the product yielded $606 million – a 31% jump over the previous year. Indicated for bipolar disorders, schizophrenia, autism and as an add-on to an antidepressant, aripiprazole was recently approved for treatment of children over 10 for bipolar disorder, and the product carries the expected atypical antipsychotic black box warnings for suicidal thoughts in children and young adults.
The antipsychotic and anti-depressant drug is the next bestselling drug after Plavix. The drug was developed by Otsuka in Japan, and it markets the drug in partnership with BMS in the U.S and Europe. The drug brought in $2.8 billion in revenues for BMS in 2012. However, BMS’s rights to commercialize the drug in EU and U.S. will end in 2014 and 2015 respectively. Simultaneously, Otsuka’s U.S. patent for the drug expires in 2014, and the drug will face competition from generic products soon.
(Glu, Ala, Lys, Tyr)x•xCH3COOH
(C5H9NO4•C3H7NO2•C6H14N2O2•C9H11NO3)x•xC2H4O2
CAS - 147245-92-9
Copaxone (glatiramer acetate injection) is a treatment for relapsing-remitting multiple sclerosis (RRMS) manufactured by Teva Pharmaceuticals. Sales reached $3.57 billion in 2011.
Teva Pharmaceuticals claims two companies are violating patents on Copaxone, glatiramer acetate for Multiple Sclerosis (MS). The original suit against Momenta Pharmaceuticals and Sandoz now has expanded to include ten patents, three of which run past the expected generic drug entry of 2014…so when generics will appear is now an open question. Copaxone, approved as an orphan drug, is Teva's flagship product, with sales of over $680 million a year. In addition to being in clinical trials for various combinations, dosage rates, and administration strategies in the mitigation of MS, the drug is now also being studied for macular degeneration and Crohn's Disease.
Teva Pharmaceuticals claims two companies are violating patents on Copaxone, glatiramer acetate for Multiple Sclerosis (MS). The original suit against Momenta Pharmaceuticals and Sandoz now has expanded to include ten patents, three of which run past the expected generic drug entry of 2014…so when generics will appear is now an open question. Copaxone, approved as an orphan drug, is Teva's flagship product, with sales of over $680 million a year. In addition to being in clinical trials for various combinations, dosage rates, and administration strategies in the mitigation of MS, the drug is now also being studied for macular degeneration and Crohn's Disease.

Gleevec (imatinib mesylate) is a treatment for chronic myeloid leukemia (CML)and gastrointestinal stromal tumor (GIST). Global sales were $4.26 billion in 2010. Gleevec is manufactured by Novartis.
| Gleevec® | imatinib | 2015-01 | exp---------Chronic myeloid leukemia, gastrointestinal stromal tumors | NOVARTIS |

Namenda (memantine HCI) is a treatment for moderate to severe Alzheimer's disease manufactured by Forest Laboratories, Inc.

Provigil (modafinil), a treatment for excessive sleepiness and shift work sleep disorder, is manufactured by Teva Pharmaceuticals. The product generated $350 million in sales in 2011.
 |
C20H30BrNO3•H2O ipratropium bromide Mol. Wt. 430.4

albuterol sulfate
Combivent (albuterol and ipratropium inhalation) is management of for chronic obstructive pulmonary disease (COPD) symptoms. Boehringer Ingelheim reported sales of $965 million in 2010.

Zyvox (linezolid) is an antiviral manufactured by Pfizer. The company reported global sales of $325 million in 2011.

Prezista (darunavir) is a protease inhibitor for human immunodeficiency virus (HIV) infection. Distributed by Janssen Therapeutics, Prezista sales were $888 million globally in 2010.

SEVELAMER
Renagal sevelamer, us dollar 400 in 2008

Avodart (dutasteride) is a treatment for benign prostatic hyperplasia (enlarged prostate gland). GlaxoSmithKline reported sales of $973 million worldwide 2010.

EFAVIRENZ
sustiva, efavirenz, US DOLLAR 200 IN 2008
Sustiva is a drug from BMS for the treatment of HIV. When sold along with another drug called Truvada* (which is developed by Gilead, an American biotechnology company) in a fixed-dose combination, the package is called Sustiva franchise. Such fixed dose combinations help simplify HIV therapy for patients and providers. BMS made over $1.5 billion in revenues from this franchise in 2012. The patent for Sustiva ends in 2013 and 2015 for E.U and U.S respectively. However, this is not likely to end the exclusivity for the combination therapy as described above. We expect a gradual decline in the sales of Sustiva as more companies develop generic variants and know how about the variants increases in the market.
..........................................................................................
(colesevelam hydrochloride)
Daiichi Sankyo
Primary Hyperlipidemia;
Type II Diabetes Mellitus
$385 million March 2015
...............................................................................................
(aprepitant)
Merck
Chemo-Associated Nausea
& Vomiting; Prevention of
Post-Op Nausea & Vomiting
$114 million April 2015
........................................................................................
LOVAZA (omega 3 fatty acids)
GlaxoSmithKline
Hypertriglyceridemia $998 million Q1 2015
Join me-Do not miss me
YEAR 2016

HUMIRA, ADALIMUMAB
Adalimumab (HUMIRA, AbbVie) is the third TNF inhibitor, after infliximab and etanercept, to be approved in the United States. Like infliximab and etanercept, adalimumab binds to Tumor necrosis factor-alpha (TNFα), preventing it from activating TNF receptors. Adalimumab was constructed from a fully human monoclonal antibody, while infliximab is a mouse-human chimericantibody and etanercept is a TNF receptor-IgG fusion protein. TNFα inactivation has proven to be important in downregulating theinflammatory reactions associated with autoimmune diseases. As of 2008 adalimumab has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, moderate to severe chronic psoriasis and juvenile idiopathic arthritis. Although only approved for ulcerative colitis from late 2012 by the FDA in the disease's management, it has been used for several years in cases that have not responded to conventional treatment at standard dosing for Crohn's Disease.
The global autoimmune market is a nearly $25 billion market and includes treatments for rheumatoid arthritis, Crohn’s disease, Addison’s disease, multiple sclerosis, and type I diabetes.
Abbott’s Humira is mainly used to treat rheumatoid arthritis and is the world’s best-selling drug in the autoimmune market with an estimated market share of well over 30%. Humira’s revenues exceeded $8 billion in 2011 and we expect it to top $10 billion by 2013. Abbott mainly competes with Pfizer and Johnson & Johnson in this market.We expect Humira’s market share to gradually decline as competition penetrates the market. However, as evident from the above chart, we expect a steep decline post-U.S. patent expiry in December 2016 (including a year’s extension).

cinacalcet, sensipar amgen
Cinacalcet (INN) is a drug that acts as a calcimimetic (i.e. it mimics the action of calcium on tissues) by allosteric activation of thecalcium-sensing receptor that is expressed in various human organ tissues. It is sold by Amgen under the trade name Sensiparin North America and Australia and as Mimpara in Europe. Cinacalcet is used to treat secondary hyperparathyroidism (elevatedparathyroid hormone levels), a consequence of end-stage renal disease.[1] Cinacalcet is also indicated for the treatment of hypercalcemia in patients with parathyroid carcinoma
B.C. Van Wagenen, S.T. Moe, M.F. Balandrin, E.G. DelMar, E.F. Nemeth, U.S. Patent 6,211,244 (2001).

Crestor (rosuvastatin calcium) by Astra Zeneca, is a treatment for lowering LDL cholesterol I the bloodstream. Crestor sales were $6 billion in 2010.

Benicar (olmesartan medoxomil) for high blood pressure is manufactured by Daiichi Sankyo, Inc. In 2010, sales were $2.5 billion.

Cubicin (daptomycin) is an anti-viral manufactured by Cubist Pharmaceuticals. The drug generated $644 million in global sales in 2010.

fluticasone propionate

Salmeterol xinafoate
ADVAIR, fluticasone propionate, Salmeterol xinafoate

fluticasone propionate

Salmeterol xinafoate
ADVAIR, fluticasone propionate, Salmeterol xinafoate
GlaxoSmithKline (GSK) may soon face generic competition for Advair, its blockbuster asthma drug. According to a September 10thWall Street Journal article, the Food and Drug Administration (FDA) has issued draft guidelines for generic versions of Advair (known as Seretide outside the U.S.). Since its FDA approval in 2000, the drug has proven to be a very lucrative product for GSK. As the company’s biggest drug by sales, Advair accounted for about 20% — which translates into $8 billion — of the company’s annual revenues from all markets. The U.S. market represents more than 50% of all Advair sales, by far the largest consumer market for this drug. Based on statistics from IMS Health, Advair sales in the U.S. have risen steadily from $4.4 billion in 2008 to $4.9 billion in 2012.
Advair is delivered by an inhaler designed to treat asthma in patients aged 4 years and older. According to the Centers for Disease Control and Prevention (CDC), the number of adults with asthma in the U.S. in 2011 was 18.9 million—that’s 8.2% of the population. Approximately 7.1 million children under age 18 have asthma, representing 9.5% of their respective population. From 2002-2007, the CDC estimated that the disorder costs $3,300 annually per person in medical expenses. Within the same time period, total national medical costs for asthma increased by 5.6% to $56 billion in 2007 from $53 billion in 2002.
The U.S. patent on Advair’s ingredients expired in 2010 and the patent on its inhaler is set to expire in 2016
.........................................................................WILLL BE UPDATED SOON KEEP WATCHING
YEAR 2017

atazanavir
Atazanavir marketed under the trade name Reyataz by Bristol Myers, (formerly known as BMS-232632) is an antiretroviral drug of the protease inhibitor (PI) class. Like other antiretrovirals, it is used to treat infection of human immunodeficiency virus (HIV).
Atazanavir is distinguished from other PIs in that it can be given once-daily (rather than requiring multiple doses per day) and has lesser effects on the patient's lipid profile (the amounts of cholesterol and other fatty substances in the blood). Like other protease inhibitors, it is used only in combination with other HIV medications.
The U.S. Food and Drug Administration (FDA) approved atazanavir on June 20, 2003. Atazanavir is the first PI approved for once-daily dosing, and also appears to be less likely to cause lipodystrophy and elevated cholesterol as side effects. It may also not be cross-resistant with other PIs. When boosted with ritonavir it is equivalent in potency to lopinavir for use in salvage therapy in patients with a degree of drug resistance, although boosting with ritonavir reduces the metabolic advantages of atazanavir.
On October 20, 2006, the FDA approved a new formulation of atazanavir (300 mg capsules) to be taken as part of combination drug therapy. This formulation should reduce pill burden, as one 300 mg capsule may replace two 150 mg capsules.
Reyataz - Reyataz is another drug for the treatment of HIV. BMS develops it under a worldwide license from Novartis and markets it as a combination with Norvir, a drug from Abbott Laboratories. The market exclusivity for the drug ends in U.S., Canada and China in 2017 and in major EU countries and Japan in 2019. The drug accounted for $1.5 billion of BMS’s 2012 revenues.
Reyataz - Reyataz is another drug for the treatment of HIV. BMS develops it under a worldwide license from Novartis and markets it as a combination with Norvir, a drug from Abbott Laboratories. The market exclusivity for the drug ends in U.S., Canada and China in 2017 and in major EU countries and Japan in 2019. The drug accounted for $1.5 billion of BMS’s 2012 revenues.
....................................................................................

Eleptripan, relpax USD 625 in 2008
| Relpax® | eletriptan | exp2017-08 | Migraines | PFIZER IRELAND |
Eletriptan Hydrobromide, CAS number 143322-58-1, is a medication indicated for the acute treatment of migraine with or without aura in adults. Known as Relpax, which is marketed by Pfizer, the patent expiration for Eletriptan Hydrobromide is on December 26, 2016 (Patent Use Code U – 876 – TREATMENT OF MIGRAINE WITH OR WITHOUT AURA). Available in 20 milligram and 40 milligram tablets, eletriptan hydrobromide has been shown to be more effectual with the higher dosage of 40 milligrams. Doses of 80 milligrams in trials were deemed efficacious, but led to a greater instance of adverse effects. The maximum recommended dose of eletriptan hydrobromide is 40 milligrams. Common side effects of eletriptan hydrobromide are dizziness, nausea, weakness, drowsiness, and pain or pressure sensations in the chest or throat.
......................................................................................

EZETIMIBE
Zetia, ezetimibe USD 1225 in 2008
.....................................................................................

byetta,exenatide. usd 627 in 2008
........................................................................................
lopinavir and ritonavir
Abbott’s Kaletra (generic lopinavir and ritonavir) is an antiviral used to treat HIV and was approved in 2000. It currently has a market share of about 8% in the $18 billion HIV antiviral market. However its market share has been declining due to competition and its exclusion from the list of preferred treatment options in some of the U.S. Department of Health and Human Services’ guidelines. Bristol-Myers’s drug atazanavir (generic reyataz) and Johnson & Johnson’s darunavir (generic prezista) are major competitors in the market.
We expect Kaletra’s market share to continue its gradual decline through 2015, and then drop sharply following its patent expiry in June 2016 to about 2.5% by the end of the Trefis forecast period.
.....................................................year 2018

Cetuximab (Erbitux) is an epidermal growth factor receptor (EGFR) inhibitor used for the treatment of metastatic colorectal cancer and head and neck cancer. Cetuximab is a chimeric (mouse/human) monoclonal antibody given by intravenous infusion that is manufactured and distributed in the United States by the drug companies Bristol-Myers Squibb and Eli Lilly and Companyand in Europe by the drug company Merck KGaA.
In July 2009, the FDA approved Erbitux for treatment of KRAS wild type colon cancer, since Cetuximab had little or no effect in colorectal tumors harboring a KRAS mutation (this also applied to the EGFR antibody panitumumab)This was the first genetic test to guide treatment of cancer. In July 2012, the FDA approved a real time PCR companion diagnostic test for KRAS, the therascreen KRAS test. Erbitux is a treatment for the cure of colorectal cancer, and head and neck cancer. BMS distributes the drug in partnership with Eli Lilly and reported $702 million in revenues from the sale of this drug. Data exclusivity for this drug ends in 2016 in the U.S., while the patent expires in 2018.
Orlistat (also known as tetrahydrolipstatin) is a drug designed to treat obesity. It is marketed as a prescription under the trade name Xenical by Roche in most countries, and is sold over-the-counter as Alli by GlaxoSmithKline in the United Kingdom and the United States. Its primary function is preventing the absorption of fats from the human diet by acting as alipase inhibitor, thereby reducing caloric intake. It is intended for use in conjunction with a healthcare provider-supervisedreduced-calorie diet
| Xenical® | Orlistat | 2018-01 | HOFFMANN LAROCHE |

| XOLEGEL® | KETOCONAZOLE | 2018-12 | AQUA PHARMS |
NIZORAL® is a synthetic broad-spectrum antifungal agent available in scored white tablets, each containing 200 mg ketoconazole base for oral administration. Inactive ingredients are colloidal silicon dioxide, corn starch, lactose, magnesium stearate, microcrystalline cellulose, and povidone. Ketoconazole is cis-1-acetyl-4-[4-[[2-(2,4dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxyl]phenyl] piperazine
email me if u like me
http://newdrugapprovals.wordpress.com/patent-expiry/
additional info
MORE INFO
http://newdrugapprovals.wordpress.com/patent-expiry/
additional info
Patent expirations up to 2016 will reduce brand spending in worldwide developed markets by $127 billion, but will be counterbalanced by generic spending, resulting in a five-year global savings of close to $106 billion. In Japan, the UK, France and Germany, many of the same or similar brands that were impacted in the U.S. will also lose patent protection. For example, in Japan, the use of generics is expected to increase as the government promotes policies to use lower-cost alternatives, and major products face generic competition. Overall, exclusivity loss in one or more developed markets will impact seven of the top 10 leading prescription drugs, including Lipitor, Plavix, Advair Diskus, Crestor, and Nexium.
Source: IMS Institute for Healthcare Informatics. The Global Use of Medicines: Outlook Through 2016. July 2012.
| Protection Expiry Year | US | Japan | UK | France | Germany |
|---|---|---|---|---|---|
| 2012 | Plavix® Seroquel® Singulair® Actos® Lexapro® Diovan® Diovan HCT® Geodon® Viagra® Boniva® | Nu Lotan Myslee® Preminent Haigou Seroquel® | Lipitor® Amias Seroquel® Aricept® Singulair® | Tahor Singulair® Pariet® Ixprim Aprovel | Seroquel® Atacand® Atacand® Plus Sortis® Aricept® |
| 2013 | Oxycontin® Aciphex® Zometa® Xeloda® Opana®ER Asacol® | Diovan® Plavix® Livalo® Elplat® | Viagra® Xeloda® | Seretide® Coaprovel Xeloda® Micardis® Viagra® | Viani® Zometa® Atmadisc® Coaprovel Viagra® |
| 2014 | Nexium® Cymbalta® Celebrex® Symbicort® Lunesta® Restasis® Evista® Sandostatin® LAR Actonel® | Prograf® Glivec® Abilify® | Abilify® Cipralex® Risperdal® Consta® | Seroplex® Abilify® Ebixa® Risperdal® Consta® LP | Axura Risperdal® Consta® Blopress Plus® |
| 2015 | Abilify® Copaxone® Gleevec® Namenda® Provigil® Combivent® Zyvox® Prezista® Avodart® | Zyprexa® Adoair® Alimta® Spiriva® Symbicort® | Spiriva® Cymbalta® Alimta® | Alimta® Spiriva® Copaxone® Protelos® Cymbalta® | Spiriva® Copaxone® Alimta® Cymbalta® |
| 2016 | Crestor® Benicar® Benicar HCT® Cubicin® | Blopress Baraclude® | Glivec® Vfend® | Glivec® Cancidas® Vfend® | Glivec® Zyvoxid Vfend® |
Off Going Patents 2013-2016
|
http://newdrugapprovals.wordpress.com/patent-expiry/

MOST POPULAR BLOG BY DR ANTHONY, INDIA