Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, helping millions with websites, 9 million hits on google, 2.5 lakh connections worldwide, P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
Tuesday, 21 January 2014
Actavis sells seven European businesses to Aurobindo
India's Aurobindo Pharma is taking over some of Actavis' operations in seven western European countries for around 30 million euros as part of its strategy to expand in the continent.
The deal gives Aurobindo "personnel, commercial infrastructure, products, marketing authorizations and dossier licenses" in France, Italy, Portugal, Netherlands, Belgium, Spain and Germany. The Hyderabad-based firm has been expanding its presence iunthe latter two countries and the UK since 2006 and the Actavis deal will it with "a top 10 position in several key markets".
Read more at: http://www.pharmatimes.com/Article/14-01-20/Actavis_sells_seven_European_businesses_to_Aurobindo.aspx#ixzz2r13wYgYE
Sunday, 19 January 2014
Vivus has presented data on its already-approved but not-yet-marketed erectile dysfunction drug Stendra which shows that the treatment is effective for sexual activity within 15 minutes.
Vivus has presented data on its already-approved but not-yet-marketed erectile dysfunction drug Stendra which shows that the treatment is effective for sexual activity within 15 minutes.

Stendra (avanafil) was given the green light by the US Food and Drug Administration over a year ago, but there has been no launch yet as Vivus has been seeking a partner. The latest data should be attractive to potential suitors and could help Stendra take on other phosphodiesterase type 5 (PDE5) inhibitors, notably Pfizer’s Viagra (sildenafil) but also Eli Lilly’s Cialis (tadalafil) and Bayer’s Levitra (vardenafil).
read all at
Avanafil can be synthesized from a benzylamine derivative and a pyrimidine derivative:Yamada, K.; Matsuki, K.; Omori, K.; Kikkawa, K.; 2004, U.S. Patent 6,797,709

- A cutting that phenanthrene by a methylthio urea ( a ) and ethoxy methylene malonate ( 2 ) cyclization of 3 , chloride, phosphorus oxychloride get 4 , 4 with benzyl amine 5 occurred SNAr the reaction product after oxidation with mCPBA 6 . In pyrimidine, if the 2 – and 4 – positions are active simultaneously the same leaving group in the case, SNAr reaction occurs preferentially at 4 – position, but does not guarantee the 2 – side reaction does not occur. Here is an activity of the poor leaving group sulfide spans 2 – bit, and a good leaving group active chlorine occupy four – position, thus ensuring a high regioselectivity of the reaction. 4 – position after completion of the reaction, then the 2 – position of the group activation, where sulfide sulfoxide better than the leaving group. Amino alcohols 7 and 6 recurrence SNAr reaction 8 , 8 after alkaline hydrolysis and acid alpha amidation get that phenanthrene.
Supervision of Chinese-Made Drug Substances by Philippe André
Supervision of Chinese-Made Drug Substances by Philippe André
Why source drug substances from China?
Large markets, economies of scale and cheaper labor;An industrial ecosystem supplying raw materials and equipment;Developed infrastructure and industry friendly policies;About 5,000 manufacturers;
Large markets, economies of scale and cheaper labor;An industrial ecosystem supplying raw materials and equipment;Developed infrastructure and industry friendly policies;About 5,000 manufacturers;
Thousands of chemists and students across China looking for novel synthesis routes for generic drug substances and intermediates.
read all at
Saturday, 18 January 2014
Drug to ‘cure’ cravings-compound found in the bark of an African bush may hold clues to the development of drugs for reversing a host of addictive behaviours from drug
Drug to ‘cure’ cravings-compound found in the bark of an African bush may hold clues to the development of drugs for reversing a host of addictive behaviours from drug
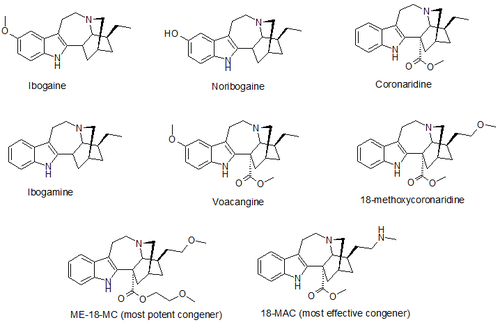
(–)-18-Methoxycoronaridine (18-MC)
A compound found in the bark of an African bush may hold clues to the development of drugs for reversing a host of addictive behaviours from drug and alcohol abuse and even smoking and compulsive over-eating, scientists reported at the BIO convention in Chicago, US, in April 2013.
read all at
 | |
|---|---|
(–)-18-Methoxycoronaridine (18-MC) is a derivative of ibogaine invented in 1996 by the research team around the pharmacologistStanley D. Glick from the Albany Medical College and the chemist Martin E. Kuehne from the University of Vermont. In animal studies it has proved to be effective at reducing self-administration of morphine, cocaine, methamphetamine, nicotine and sucrose. 18-MC is a selective α3β4 nicotinic antagonist and, in contrast to ibogaine, has no affinity at the α4β2 subtype nor at NMDA-channels nor at the serotonin transporter, and has significantly reduced affinity for sodium channels and for the σ receptor, but retains modest affinity for the μ and κ opioid receptors. The sites of action in the brain include the medial habenula, interpeduncular nucleus, dorsolateral tegmentum and basolateral amygdala. It has also been shown to produce anorectic effects in obese rats, most likely due to the same actions on the reward system which underlie its anti-addictive effects against drug addiction.
18-MC has not yet been tested in humans. In 2002 the research team started trying to raise funds for human trials, but were unable to secure the estimated $5 million needed. Efforts to raise funds for future trials are still ongoing. In January 2010, Obiter Research, a chemical manufacturer in Champaign, Illinois, signed a patent license with Albany Medical College and the University of Vermont allowing them the right to synthesize and market 18-MC and other congeners.
A number of derivatives of 18-MC have also been developed, with several of them being superior to 18-MC itself, the methoxyethyl congener ME-18-MC being more potent than 18-MC but with similar efficacy, and the methylamino analogue 18-MAC being more effective than 18-MC but with around the same potency. These compounds were also found to act as selective α3β4 nicotinic acetylcholine antagonists, with little or no effect on NMDA receptors.
BY WORLD DRUG TRACKER
Subscribe to:
Comments (Atom)