Cabotegravir, GSK 744 IN PHASE 2 FOR HIV INFECTION « New Drug Approvals: "
Cabotegravir, GSK 744,
(3S,11aR)-N-(2,4-Difluorobenzyl)-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydro[1,3]oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide
3S, 1 1 aR)- N-[(2,4-difluorophenyl)methyl]-2,3,5,7, 1 1 , 1 1 a-hexahydro-6-hydroxy-3- methyl-5,7- dioxo-oxazolo[3,2-a]pyrido[1 ,2-d]pyrazine-8-carboxamide"
'via Blog this'
Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, helping millions with websites, 9 million hits on google, 2.5 lakh connections worldwide, P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
Thursday, 10 April 2014
Cabotegravir, GSK 744 IN PHASE 2 FOR HIV INFECTION « New Drug Approvals
Cabotegravir, GSK 744 IN PHASE 2 FOR HIV INFECTION « New Drug Approvals: "
Cabotegravir, GSK 744,
(3S,11aR)-N-(2,4-Difluorobenzyl)-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydro[1,3]oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide
3S, 1 1 aR)- N-[(2,4-difluorophenyl)methyl]-2,3,5,7, 1 1 , 1 1 a-hexahydro-6-hydroxy-3- methyl-5,7- dioxo-oxazolo[3,2-a]pyrido[1 ,2-d]pyrazine-8-carboxamide"
'via Blog this'
Cabotegravir, GSK 744,
(3S,11aR)-N-(2,4-Difluorobenzyl)-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydro[1,3]oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide
3S, 1 1 aR)- N-[(2,4-difluorophenyl)methyl]-2,3,5,7, 1 1 , 1 1 a-hexahydro-6-hydroxy-3- methyl-5,7- dioxo-oxazolo[3,2-a]pyrido[1 ,2-d]pyrazine-8-carboxamide"
'via Blog this'
Tuesday, 8 April 2014
Sun Pharma has bought Ranbaxy for $4 billion to create the world’s fifth-biggest generic drugmaker.
 Dilip sanghvi, sun pharma promoter
Dilip sanghvi, sun pharma promoter
The move will make the company the largest pharma firm in India, while Daiichi Sankyo – majority owner of Ranbaxy – will become the second largest shareholder in Sun Pharma with a 9% stake and the right to nominate one director to Sun Pharma’s Board of Directors. http://www.pharmatimes.com/Article/14-04-07/Sun_buys_Ranbaxy_for_4_billion.aspx
Read more at: http://www.pharmatimes.com/Article/14-04-07/Sun_buys_Ranbaxy_for_4_billion.aspx#ixzz2yGIjkMob



Dilip Shanghvi, Managing Director of Sun Pharma said in a release, “Ranbaxy has a significant presence in the Indian pharma market and in the US where it offers a broad portfolio of ANDAs and first-to-file opportunities. In high-growth emerging markets, it provides a strong platform which is highly complementary to Sun Pharma’s strengths,”
Under the agreement, Ranbaxy shareholders will get 0.8 shares of Sun Pharma for each Ranbaxy share.
Arun Sahwney, managing director and chief executive officer of Ranbaxy said in a statement, “Sun Pharma has a proven track record of creating significant long-term shareholder value and successfully integrating acquisitions into its growing portfolio of assets,”
Who Will Benefit?
Daiichi Sankyo Co. Ltd is the parent company of Ranbaxy as they acquired it from previous promoters and investors. As soon as Ranbaxy was acquired, their plants came under a scanner from US Food and Drug Administration (FDA), which troubled Daiichi as their own reputation was under stake.
Now, they will be the most relived entity as Sun Pharma will manage all such cases pertaining to Ranbaxy. Daiichi will now control 9% of Sun Pharma as a result of the current acquisition.
Insiders are claiming that Daiichi will sell this 9% stake as well and come out of the business all together.
Ranbaxy shareholders have cheered this latest development as their shares have gained since the announcement of this deal.
Buserelin a luteinizing hormone-releasing hormone (LHRH) agonist
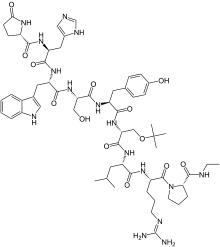
Buserelin
57982-77-1 cas no
(2S)-N-[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2R)-1-[[(2S)-1-[[(2S)-5-(diaminomethylideneamino)-1-[(2S)-2-(ethylcarbamoyl)pyrrolidin-1-yl]-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-[(2-methylpropan-2-yl)oxy]-1-oxopropan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]-5-oxopyrrolidine-2-carboxamide
6-[O-(1,1-dimethylethyl)-D-serine]-9-(N-ethyl-L-prolinamide)-10-deglycinamideluteinizing hormone-releasing factor (pig)
Profact, 57982-77-1, Buserelin (INN), Tiloryth (TN), AC1Q5OOQ, AC1L18ON, D-Ser(Tbu)6EA10LHRH,
Molecular Formula: C60H86N16O13
Molecular Weight: 1239.42424
Therap-Cat: Antineoplastic (hormonal). Gonad-stimulating principle.
Therap-Cat-Vet: Gonad-stimulating principle.
Keywords: Antineoplastic (Hormonal); LH-RH Analogs; Gonad-Stimulating Principle; LH-RH Agonist.

Buserelin is a luteinizing hormone-releasing hormone (LHRH) agonist, a synthetic hormone which stimulates the pituitary gland’s gonadotrophin-releasing hormone receptor (GnRHR). It is used in prostate cancer treatment.
Buserelin stimulates the pituitary gland's gonadotrophin-releasing hormone receptor (GnRHR). Buserelin desensitizes the GnRH receptor, reducing the amount of LH and testosterone. However, there is a concomitant surge in LH and testosterone levels with the decrease in androgens, so antiandrogens must administered.
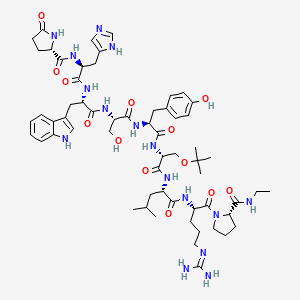 buserelin
buserelin
Properties: [a]D20 -40.4° (c = 1 in dimethylacetamide).
Optical Rotation: [a]D20 -40.4° (c = 1 in dimethylacetamide)
Derivative Type: Monoacetate
CAS : 68630-75-1
Codes: HOE-766
Trademarks: Receptal (Intervet); Suprecur (Sanofi-Aventis); Suprefact (Sanofi-Aventis)
MF: C60H86N16O13.C2H4O2
MW: 1299.48
Percent Composition: C 57.30%, H 6.98%, N 17.25%, O 18.47%
Buserelin is a Gonadotropin-releasing hormone agonist (GnRH agonist). The drug's effects are dependent on the frequency and time course of administration. GnRH is released in a pulsatile fashion in the postpubertal adult. Initial interaction of any GnRH agonist, such as buserelin, with the GnRH receptor induces release of FSH and LH by gonadotrophes. Long-term exposure to constant levels of buserelin, rather than endogenous pulses, leads to downregulation of the GnRH receptors and subsequent suppression of the pituitary release of LH and FSH.
Like other GnRH agonists, buserelin may be used in the treatment of hormone-responsive cancers such as prostate cancer or breast cancer, estrogen-dependent conditions (such as endometriosis or uterine fibroids), and in assisted reproduction.
It is normally delivered via a nasal spray, but is also available as an injection.
Buserelin acetate is marketed by Sanofi-Aventis under the brand name Suprefact and a generic form of Buserelin is now produced by CinnaGen under the brand name CinnaFact.
Buserelin is also marketed under the brand name Metrelef. Metrelef is approved to treat patients with endometriosis by suppression of ovarian hormone production. In ovulation induction Metrelef is used as a pituitary blockade as an adjunct togonadotrophin administration.
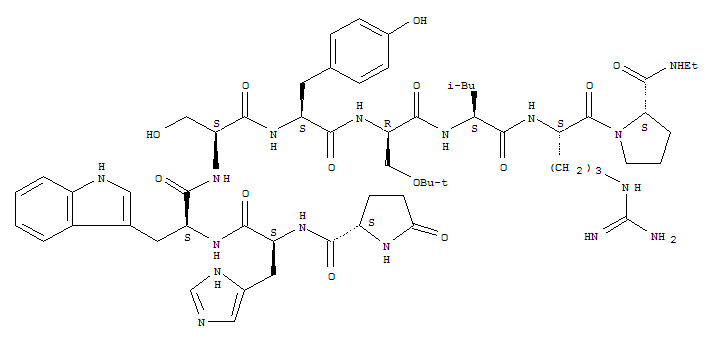
Buserelin, a synthetic gonadotropin-releasing hormone (GRH) agonist, specifically binds to GRH receptor presented at anter iorpituitary and increases or decreases the number of receptors in hypophysis through auto- regulation mechanism (G. Tolis et al., Tumor Growth Inhibition in Patients with Prostatic Carcinoma Treated with Luteinizing Hormone-Feleasing Hormone Agonists, Proc. Natl. Acad. Sci. , 79, pl658, 1982).
<5> The synthetic methods for preparing peptides are divided into two methods, i.e., liquid phase synthesis and solid phase synthesis. The liquid phase peptide synthesis of which all the reagents reacts together under the solution phase by being dissolved in the solution, has been reported to show rapid reaction rate however it has disadvantages such as the difficulty in separating and purification of the products. In a while, solid phase peptide synthesis which have been developed based on the theory of R. B. Merrifield, has been reported to have various advantages comparing with the former method for example, convenient to isolation and purification, the 'applicability to automation (Bodanszky et al, In Peptide Synthesis, John Wiley & Sons, 1976). Lots of peptide synthetic resins have been developed to synthesize various peptides after the publication of the theory of R. B. Merrifield till now. For example, chloromethyl polystyrene resin had been developed by Merrifield and Wang resin having 4-alkoxybenzyl alcohol had been developed with modifying the former resin to overcome the disadvantages thereof at the early stage. Various resins to improve the disadvantages of conventional resins have been developed after then and the representative resins among those resins are trityl group introduced 2-chlorotrityl resin and rink amide resin which can provide amide group from the carboxyl terminal of peptide under mild cleavage condition, respectively.
<6> At the early stage, the simple structured type-peptides have been synthesized using by the resins however the complex structured type peptides showing various physiological activities have been synthesized mainly. The peptides comprising unnatural amino acids have been synthesized by chemical synthetic method since the peptides could not be prepared by enzymatic synthesis. Among them, the peptides comprising D-amino acid or aza-amino acid have been reported to have potent physiological activities and further to be developed as a medicine (USP Nos. 6,624,290; 6,069,163; 5,965,538; and 4,634,715). However, the novel method for preparing LH-RH such as goserelin or GnRH peptides using by solid phase synthesis has been still need till now since previously known methods, for example, the methods disclosed in USP No. 5,602,231; EP No. 0518655; USP No. 6,879,289; and USP No. 3,914,412, have been reported to have unsolved problems such as a limit to obtain pure product etc.
http://www.google.com/patents/WO2008044890A1?cl=en
Example 4: Preparation of buserelin
<98> Ig of 2-chlroro trityl chloride resin showing 0.9 mM/g of substitution rate was swollen with 10ml of DMF and the reaction mixture mixed with 768 mg of Fmoc-Arg (N02)-0H (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto to react together. The resulted resin was treated with 20% piperidine to remove the Fmoc residue and the reaction mixture mixed with 615 mg of Fmoc-Leu-OH (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto to react together with a similar way to the above-described method. After washing the resin, the resulted resin was treated with 20% piperidine to remove the Fmoc residue and the reaction mixture mixed with 670 mg of Fmoc-D- SeKtBu)-OH (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto again to react together with a similar way to the above-described method. After washing the resin, the resulted resin was treated with 20% piperidine to remove the Fmoc residue and the reaction mixture mixed with 859 mg of Fmoc-Tyr(OBzI)-OH (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto again to react together with a similar way to the above-described method. After washing the resin, the resulted resin was treated with 20% piperidine to remove the Fmoc residue and the reaction mixture mixed with 726 mg of Fmoc-Ser(OBzI)-OH (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto again to react together with a similar way to the above- described method. After washing the resin, the resulted resin was treated with 20% piperidine to remove the Fmoc residue and the reaction mixture mixed with 742 mg of Fmoc-Trp-0H (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto again to react together with a similar way to the above- described method. After washing the resin, the resulted resin was treated with 20% piperidine to remove the Fmoc residue and the reaction mixture mixed with 1.078g of Fmoc-His(Fmoc)-0H (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto again to react together with a similar way to the above-described method. After washing the resin, the resulted resin was treated with 20% piperidine to remove the Fmoc residue and the reaction mixture mixed with 244 mg of Pyr-OH (1.74 mM) and 271 microliter of DIC (1.74 mM) was added thereto again to react together with a similar way to the above-described method.
<99> The resin was washed again and 2ml of 1% TFA (Trifluoroacetic acid)/DCM (dichloromethane) per 70mg of peptide resin was added to the resin, eluted to release the peptide from the resin and the elute was collected with 200 microliter of pyridine. The above-described step was repeated five times. The resin was washed with DCM (dichloromethane) and methanol and the elute was collected with the former elute. The elute was concentrated with evaporation and ether was added thereto to obtain the precipitated peptide. The precipitated peptide was performed to coupling reaction with 305 mg of Pro- NH-CH2CH3 (2.4mM) and 303mg of DIC (2.4 niM) in the presence of DCM
(dichloromethane) solvent. The solution was subjected to concentration with evaporator. The resulting concentrate was dissolved in EtOAc, washed with saturated NaHCOs solution, distilled water, 5% citrate solution and dried with anhydrous MgS(V The remaining MgS04 was discarded with filtration and the filtrate was concentrated with evaporation. The benzyl group and Cbz group among the side chain protecting group in the peptide were removed through catalytic hydrogen transfer reaction using by Pd/C and ammonium formate in the presence of methanol. The resulting peptide was purified with reverse phase column chromatography (Shimadzu H-kit, acetonitrile^water= 22:78 → 32:68, 1% increase/min) to isolate pure buserelin (Yield: 40%).
new patent
WO-2014047822
Solid state method for the preparation of buserelin, an LHRH analog useful for the treatment of sexual dysfunction, ovulation, puberty retardation and cancer. Method is under basic conditions and increases yield and purity. This appears to be the first PCT application from Hybio with this target, however several Chinese national filings have been published. Pan, Ma and Yuan are named on several previous solid phase synthesis PCT applications, most recently WO2013117135.
References:
Synthetic nonapeptide agonist analog of LH-RH, q.v. Synthesis: W. Konig et al., DE 2438350; eidem, US4024248 (1976, 1977 both to Hoechst);
A. S. Dutta et al., J. Med. Chem. 21, 1018 (1978).
Clinical pharmacology: A. Lemay et al.,Fertil. Steril. 37, 193 (1982).
Radioimmunoassay in plasma and urine: S. Saito et al., J. Immunol. Methods 79, 173 (1985).
Veterinary use to increase conception rate: K. Moller, E. D. Fielden, N. Z. Vet. J. 29, 214 (1981).
Clinical evaluation in prostatic carcinoma: J. H. Waxman, Br. J. Urol. 55, 737 (1983); as ovulatory stimulant for in vitro fertilization: V. MacLachlan et al., N. Engl. J. Med. 320, 1233 (1989).
Review of pharmacokinetics and clinical profile: R. N. Brogden et al., Drugs 39, 399-437 (1990); of efficacy in prostatic carcinoma: H. J. de Voogt et al., Scand. J. Urol. Nephrol. Suppl 138, 131-136 (1991).
| US5212288 * | Feb 8, 1991 | May 18, 1993 | Syntex (U.S.A.) Inc. | Temporary minimal protection synthesis of serine-containing polypeptides |
| US5510460 * | May 26, 1995 | Apr 23, 1996 | Zeneca Limited | Peptide process |
| US5602231 * | May 26, 1995 | Feb 11, 1997 | Zeneca Limited | Process for making peptides |
| US6028172 * | Feb 10, 1998 | Feb 22, 2000 | Mallinckrodt Inc. | Reactor and method for solid phase peptide synthesis |
| US6897289 * | May 5, 2000 | May 24, 2005 | Lipotec, S.A. | Peptide synthesis procedure in solid phase |
Subscribe to:
Comments (Atom)