ET-743, Yondelis (trabectedin)
Trabectedin, Ecteinascidin 743, NSC-684766, ET-743, Yondelis, ID0YZQ2TCP
cas 114899-77-3
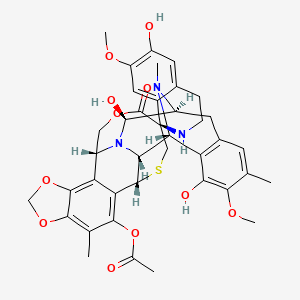
(-)-(1’R,6R,6aR,7R,13S,14S,16R)-5-Acetoxy-6′,8,14-trihydroxy-7′,9-dimethoxy-4,10,23-trimethyl-1′,2′,3′,4′,6a,7,12,13,14,16-decahydro-6H-spiro[6,16-(epithiopropanoxymethano)-7,13-epimino-1,3-dioxolo[7,8]isoquino[3,2-b][3]benzazocine-20,1′-isoquinolin]-19-one
Janssen seeks FDA approval for Yondelis drug to treat advanced STS
Janssen Research & Development is seeking approval from US Food and Drug Administration (FDA) for its Yondelis (trabectedin) to treat patients with advanced soft tissue sarcoma (STS).
Trabectedin, also referred as ET-743 during its development, is a marine derived antitumoral agent discovered in the Carribean tunicate _Ecteinascidia turbinata_ and now produced synthetically. Trabectedin has a unique mechanism of action. It binds to the minor groove of DNA interfering with cell division and genetic transcription processes and DNA repair machinery.It is approved for use in Europe, Russia and South Korea for the treatment of advanced soft tissue sarcoma. It is also undergoing clinical trials for the treatment of breast, prostate, and paediatric sarcomas. The European Commission and the U.S. Food and Drug Administration (FDA) have granted orphan drug status to trabectedin for soft tissue sarcomas and ovarian cancer.
Trabectedin (also known as
ecteinascidin 743 or
ET-743) is an anti-tumor drug. It is sold by
Zeltia and
Johnson and Johnson under the brand name
Yondelis. It is approved for use in Europe, Russia and South Korea for the treatment of advanced
soft tissue sarcoma. It is also undergoing clinical trials for the treatment of breast, prostate, and paediatric sarcomas. The European Commission and the
U.S. Food and Drug Administration (FDA) have granted
orphan drug status to trabectedin for soft tissue sarcomas and
ovarian cancer.
Discovery and development
The ecteinascidins (herein abbreviated ETs) are exceedingly potent antitumor agents isolated from the marine tunicate Ecteinascidia turbinata. Several ecteinascidins have been reported previously in the patent and scientific literature. See, for example U.S. Pat. No. 5,089,273, which describes novel compounds of matter extracted from the tropical marine invertebrate Ecteinascidia turbinata, and designated therein as ecteinascidins 729, 743, 745, 759A, 759B and 770. These compounds are useful as antibacterial and/or antitumor agents in mammals. U.S. Pat. No. 5,478,932 describes other novel ecteinascidins isolated from the Caribbean tunicate Ecteinascidia turbinata, which provide in vivo antitumor activity against P388 lymphoma, B16 melanoma, M5076 ovarian sarcoma, Lewis lung carcinoma, and the LX- I human lung and MX- 1 human mammary carcinoma xenografts.
One of the ETs, ecteinascidin 743 (ET-743), is a tetrahydroisoquinoline alkaloid with considerable in vitro and in vivo antitumor activity in murine and human tumors, and potent antineoplastic activity against a variety of human tumor xenografts grown in athymic mice, including melanoma, ovarian and breast carcinoma.
ET-743 is a natural compound with the following structure:
ET-743 is also known with the generic name trabectedin and the trademark Yondelis®, and it is currently approved in Europe for the treatment of soft tissue sarcoma. The clinical development of trabectedin continues in phase 11/ III clinical trials in breast, ovarian and prostate cancer. A clinical development program of ET-743 in cancer patients was started with phase I studies investigating 1- hour, 3-hour, 24-hour, and 72-hour intravenous infusion schedules and a 1 hour daily x 5 (dx5) schedule. Promising responses were observed in patients with sarcoma, breast and ovarian carcinoma.
Therefore this new drug is currently under intense investigation in several phase 11/ III clinical trials in cancer patients with a variety of neoplastic diseases. Further information regarding the dosage, schedules, and administration of ET-743 for the treatment of cancer in the human body, either given alone or in combination is provided in WO 00/69441 , WO 02/36135, WO 03/39571 , WO 2004/ 105761 , WO 2005/039584, WO 2005/049031 , WO 2005/049030, WO 2005/049029, WO 2006/046080, WO 2006/005602, and PCT/US07/98727, which are incorporated by reference herein in their entirety.
A review of ET-743, its chemistry, mechanism of action and preclinical and clinical development can be found in Kesteren, Ch.
Van et al., Anti-Cancer Drugs, 2003, 14 (7), 487-502: “ET-743 (trabectedin, ET-743): the development of an anticancer agent of marine origin”, and references therein.
During the past 30 years medical oncologists have focused to optimise the outcome of cancer patients and it is just now that the new technologies available are allowing to investigate polymorphisms, gene expression levels and gene mutations aimed to predict the impact of a given therapy in different groups of cancer patients to tailor chemotherapy. Representative examples include the relationship between the Thymidylate Synthase (TS) mRNA expression and the response and the survival with antifolates, beta tubulin III mRNA levels and response to tubulin interacting agents, PTEN gene methylation and resistance to CPT- I l and, STAT3 over expression and resistance to Epidermal Growth Factor (EGF) interacting agents.
A molecular observation of potential clinical impact relates to the paradoxical relation between the efficiency of the Nucleotide Excision Repair (NER) pathway and the cytotoxicity of ET-743. In fact, tumour cells that are efficient in this DNA repair pathway appear to be more sensitive to ET-743. This evidence is in contrast with the pattern noted with platin based therapeutic regimens which are highly dependent on the lack of activity of this repair pathway (ie. an increase in ERCCl expression has been associated to clinical resistance to platinum-based anti-cancer therapy).
There are evidences on the key role of NER pathways on the cytotoxicity of ET-743 in cell lines. ET-743 binds to G residues in the minor groove of DNA forming adducts that distort the DNA helix structure and they are recognised by NER mechanisms (Pourquier, P. et al., 2001 , Proceedings of the American Association for Cancer Research Annual Meeting, Vol. 42, pp. 556. 92nd Annual Meeting of the American Association for Cancer Research. New Orleans, LA, USA. March 24-28, 2001. ISSN: 0197-016X). Takebayasi et al. (Nature Medicine, 2001 , 7(8), 961-966) have proposed that the presence of these DNA adducts in transcribed genes, blocks the Transcription Coupled NER (TC-NER) system by stalling the cleavage intermediates and producing lethal Single Strand Breaks (SSBs). It is known from Grazziotin et al (Proc.Natl.Acad.Sic.USA, 104: 13062- 13067) that the DNA adducts formed by exposure to ET-743 are transformed into double strand DNA breaks.
The fact that NER mediates ET-743 ‘s cytotoxicity has also been found in the yeast Saccharomyces cerevisae by Grazziotin et al. (Biochemical Pharmacology, 2005, 70, 59-69) and in the yeast Schizosaccharomyces pombe by Herrero et al. (Cancer Res. 2006, 66(16), 8155-8162).
In addition, Bueren et al. (Proceedings AACR Annual Meeting 2007, Abstract no. 1965) have been shown that ET-743 induces double-strand breaks in the DNA in early S phase that are detected and repaired by the Homologous Recombination Repair (HRR) pathway. In addition, Erba et al (Eur. J. Cancer, 2001 , 37(1), 97- 105) and Bueren et al (Proceedings AACR Annual Meeting 2007, Abstract no. 1965) have shown that inactivation/ mutations of genes related to the Double Strand Break detection such as DNA-PK, ATM and ATR and of genes related to Homologous Recombination Repair pathway, such as Fanconi Anemia genes, BRCAl , BRCA2 and RAD51 make cells more sensitive to trabectedin. Such unique finding is the opposite to the pattern with conventional DNA interacting agents, like in the case of microtubule poisons such as taxanes and vinorelbine.
Finally, pharmacogenomic studies prior have demonstrated that increased expression of the NER genes ERCCl and XPD in the tumor tissue does not impact the outcome of patients treated with
ET-743. However, the low expression of BRCAl in the tumor tissue is correlated with a better outcome in cancer patents treated with
ET-743. Further information can be found in WO 2006/005602, which is incorporated by reference herein in its entirety.
Three rare, autosomal recessive inherited human disorders are associated with impaired NER activity: xeroderma pigmentosum (XP), Cockayne Syndrome (CS), and trichothiodystrophy (Bootsma et al. The Genetic Basis of Human Cancer. McGraw-Hill, 1998, 245- 274). XP patients exhibit extreme sensitivity to sunlight, resulting in a high incidence of skin cancers (Kraemer et al. Arch. Dermatol. 123, 241-250, and Arch. Dermatol. 130, 1018- 1021). About 20% of XP patients also develop neurologic abnormalities in addition to their skin problems. These clinical findings are associated with cellular defects, including hypersensitivity to killing and mutagenic effects of UV, and inability of XP cells to repair UV-induced DNA damage (van Steeg et al. MoI. Med. Today, 1999, 5, 86-94).
Seven different NER genes, which correct seven distinct genetic XP complementation groups (XPA-XPG), have been identified (Bootsma et al. The Genetic Basis of Human Cancer. McGraw-Hill, 1998, 245-274). The human gene responsible for XP group G was identified as ERCC5 (Mudgett et al. Genomics, 1990, 8, 623-633; O’Donovan et al. Nature, 1993, 363, 185- 188; and Nouspikel et al. Hum. MoI. Genet. 1994, 3, 963-967). The XPG gene codes for a structure-specific endonuclease that cleaves damaged DNA ~5 nt 3′ to the site of the lesion and is also required non-enzymatically for subsequent 5′ incision by the XPF/ ERCCl heterodimer during the NER process (Aboussekhra et al. Cell, 1995, 80, 859-868; Mu et al. J. Biol. Chem. 1996, 271 , 8285-8294; and Wakasugi et al. J. Biol. Chem. 1997, 272, 16030- 16034). There is also evidence suggesting that XPG is also involved in transcription-coupled repair of oxidative DNA lesions (Le Page et al. Cell, 101 , 159- 171).
Takebayashi et al. (Cancer Lett., 2001 , 174: 1 15- 125) have observed an increase in heterozygosity loss and microsatellite instability in a substantial percentage of samples of ovarian, lung and colon carcinoma. Le Moirvan et al, (Int.J. Cancer, 2006,1 19: 1732- 1735) have described the presence of polymorphisms in the XPG gene in sarcoma patients. It is also known from Takebayashi et al. (Proceedings of the American Association forCancer Research Annual Meeting, March, 2001 , Vol. 42, pp. 813.92nd Annual Meeting of the American Association for Cancer
Research. New Orleans, LA, USA. March 24-28, 2001) that cells deficient in the NER system are resistant to treatment with ET-743 (Zewail-Foote, M. et al., 2001 , Chemistry and Biology, 8: 1033- 1049 and Damia, G. et al., 2001 , Symposium AACR NCI EORTC) and that the antiproliferative effects of ET-743 require a functional XPG gene.
Since cancer is a leading cause of death in animals and humans, several efforts have been and are still being undertaken in order to obtain an antitumor therapy active and safe to be administered to patients suffering from a cancer. Accordingly, there is a need for providing additional antitumor therapies that are useful in the treatment of cancer.
Trabectedin is a tetrahydroisoquinoline, a novel marine-derived antitumor agent isolated from the colonial tunicate Ecteinascidia turbinate. The drug binds to the minor groove of the DNA, bending the DNA towards the major groove, blocking the activation of genes in a unique way via several pathways, including selective inhibition of the expression of key genes (including oncogenes) involved in cell growth and drug resistance, inhibition of genetic repair pathways and inhibition of cell cycle progression leading to p53-independent programmed cell death.
In July 2003, the European Committee of Proprietary Medicinal Products (CPMP) recommended against granting marketing authorization to trabectedin for soft tissue sarcoma. PharmaMar appealed the decision in September 2003. Later that year, the CPMP rejected the company’s appeal. In 2006, the company filed another regulatory application for this indication and, finally, in 2007, a positive opinion was received in the E.U. for the treatment of metastatic soft tissue sarcoma. First commercialization of the product in the E.U. took place in October 2007 in the U.K. and Germany.
The compound is also available in several other countries. In 2008, the compound was filed for approval in the U.S. and the E.U. for the treatment of relapsed advanced ovarian cancer in combination with liposomal doxorubicin, and in 2009 approval was received in both countries. Trabectedin is available in several European countries, including the U.K. and Germany. Also in 2009 the drug candidate was approved in Philippines for the ovarian cancer indication.
The compound had been in phase II development by Johnson & Johnson for the treatment of prostate cancer; however, no recent development has been reported for this research. PharmaMar is evaluating the compound in phase II trials for the treatment of breast cancer. Additional early clinical trials are ongoing at the National Cancer Institute (NCI) to evaluate trabectedin for potential use in the treatment of advanced, persistent or recurrent uterine leiomyosarcomas and solid tumors.
In 2011, a regulatory application that had been filed in the U.S. seeking approval for the treatment of relapsed advanced disease in combination with liposomal doxorubicin was withdrawn by the company based on the FDA’s recommendation that an additional phase III study be conducted to obtain approval. In 2014, Janssen Research & Development, LLC submitted an NDA for trabectedin to the FDA for the treatment of patients with advanced soft tissue sarcoma (STS), including liposarcoma and leiomyosarcoma subtypes, who have received prior chemotherapy including an anthracycline.
Trabectedin was developed by PharmaMar, a subsidiary of Zeltia. The drug was being codeveloped and comarketed in partnership with Ortho Biotech, a subsidiary of Johnson & Johnson pursuant to an agreement signed in 2001. However, in 2008 the license agreement between the two companies was terminated.
The compound was granted orphan drug designation for the treatment of soft tissue sarcoma and for the treatment of ovarian cancer by the FDA and the EMEA. In 2011, orphan drug designation was granted in Japan for the treatment of malignant soft tissue tumor accompanied with chromosomal translocation. In 2009, the product was licensed to Taiho by PharmaMar in Japan for the treatment of cancer.
During the 1950s and 1960s, the
National Cancer Institute carried out a wide ranging program of screening plant and marine organism material. As part of that program extract from the
sea squirt Ecteinascidia turbinata was found to have anticancer activity in 1969.
[1]Separation and characterisation of the active molecules had to wait many years for the development of sufficiently sensitive techniques, and the structure of one of them, Ecteinascidin 743, was determined by KL Rinehart at the University of Illinois in 1984.
[2]Rinehart had collected his sea squirts by scuba diving in the reefs of the West Indies.
[3]
Recently, the biosynthetic pathway responsible for producing the drug, has been determined to come from
Candidatus Endoecteinascidia frumentensis, a microbial symbiont of the tunicate.
[4] The Spanish company
PharmaMar licensed the compound from the University of Illinois before 1994 and attempted to farm the sea squirt with limited success.
[3]
Yields from the sea squirt are extremely low – it takes 1
tonne of animals to isolate 1 gram of trabectedin – and about 5 grams were believed to be needed for a clinical trial
[5] so Rinehart asked the Harvard chemist
E. J. Corey to search for a synthetic method of preparation. His group developed such a method and published it in 1996.
[6] This was later followed by a simpler and more tractable method which was patented by Harvard and subsequently licensed to PharmaMar.
[3] The current supply is based on a semisynthetic process developed by PharmaMar starting from
Safracin B, an antibiotic obtained by fermentation of the bacterium Pseudomonas fluorescens.
[7] PharmaMar have entered into an agreement with Johnson and Johnson to market the compound outside Europe.
Trabectedin was first dosed in humans in 1996.In 2007, the EMEA gave authorisation for the marketing of trabectedin, under the trade name Yondelis, for the treatment of patients with advanced soft tissue sarcoma, after failure of
anthracyclines and
ifosfamide, or who are unsuited to receive these agents. The agency’s evaluating committee, the
CHMP observed that trabectedin had not been evaluated in an adequately designed and analyzed randomized trial against current best care, and that the clinical efficacy data was mainly based on patients with
liposarcoma and
leiomyosarcoma. However the pivotal study did show a significant difference between two different trabectedin treatment regimens, and due to the rarity of the disease the CHMP considered that marketing authorisation could be granted under exceptional circumstances.
[8] As part of the approval PharmaMar agreed to conduct a further trial to identify whether any specific chromosomal
translocations could be used to predict responsiveness to trabectedin.
[9] Trabectedin is also approved in South Korea
[10] and Russia.
In 2008 the submission was announced of a registration dossier to the
European Medicines Agency (EMEA) and the FDA for Yondelis when administered in combination with
pegylated liposomal doxorubicin (Doxil, Caelyx) for the treatment of women with relapsed
ovarian cancer. In 2011, Johnson&Johnson voluntarily withdrew the submission in the United States following a request by the FDA for an additional Phase III study to be done in support of the submission.
[11]
Trabectedin is also in phase II trials for prostate, breast and paediatric cancers.
[12]
Structure
Biosynthesis
The biosynthesis of Trabectedin in
Candidatus Endoecteinascidia frumentensis starts with a fatty acid loading onto the acyl-ligase domain of the EtuA3 module. A cysteine and glycine are then loaded as canonical NRPS amino acids. A tyrosine residue is modified by the enzymes EtuH, EtuM1, and EtuM2 to add a hydroxyl at the meta position of the phenol, and adding two methyl groups at the para-hydroxyl and the meta carbon position. This modified tyrosine reacts with the original substrate via a Pictet-Spangler reaction, where the amine group is converted to an imine by deprotonation, then attacks the free aldehyde to form a carbocation that is quenched by electrons from the methyl-phenol ring. This is done in the EtuA2 T-domain. This reaction is done a second time to yeid a dimer of modified tyrosine residues that have been further cyclized via Pictet-spangler reaction, yielding a bicyclic ring moiety. The EtuO and EtuF3 enzymes continue to post-translationally modify the molecule, adding several functional groups and making a sulfide bridge between the original cysteine residue and the beta-carbon of the first tyrosine to form ET-583, ET-597, ET-596, and ET-594 which have been previously isolated.
[4] A third o-methylated tyrosine is added and cyclized via Pictet-Spangler to yield the final product.
[4]
Proposed biosynthetic scheme for the biosynthesis of Trabecteden (ET-743)
Synthesis
Org Lett 2000,2(7),993
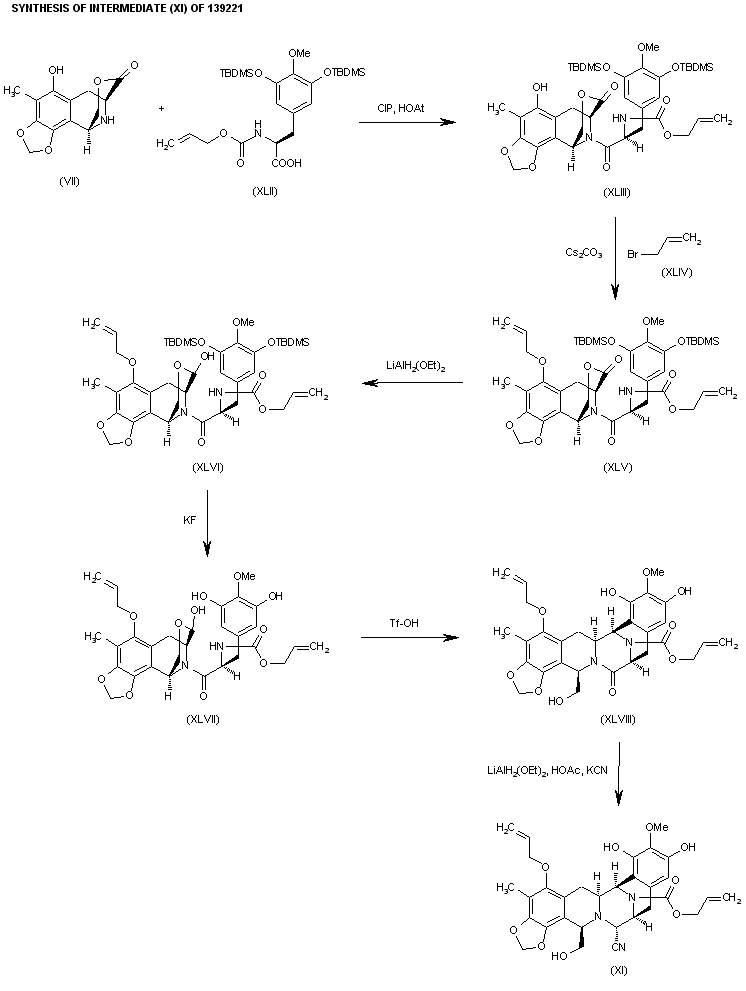
The previously reported synthesis of 139221 (scheme 13922101a) has been investigated in order to find a more efficient, reproducible and economical route to work in the mutikilogram scale. Herein it is reported a new process which is simpler and proceeds with an overall yield of 54% (the original process, 35%). The condensation of intermediate aminolactone (I) (scheme 13922101a, intermediate (VII)) with acid (XLII) (the acid derived from scheme 13922101a, intermediate ester (IX)) by means of 2-chloro-1,3-dimethylimidazolidinium hexafluorophosphate (CIP), and 1-hydroxy-7-azabenzotriazole (HOAt) in THF/dichloromethane gives the coupling product (XLIII), which is allylated with allyl bromide (XLIV) and Cs2CO3 in DMF yielding the allyl ether (XLV). The reduction of the lactone group of (XLV) with LiAlH2(OEt)2 in ethyl ether affords the lactol (XLVI), which is desilylated with KF in methanol to provide the phenolic compound (XLVII). The opening of the lactol ring of (XLVII) with simultaneous cyclization by means of Tf-OH in water/trifluoroethanol gives the hexacyclic intermediate (XLVIII), which is finally reductocondensed with KCN by means of LiAlH2(OEt)2 in THF to furnish the previously reported pentacyclic intermediate (XI) (scheme 13922101a, intermediate (XI)).
……………………………………………
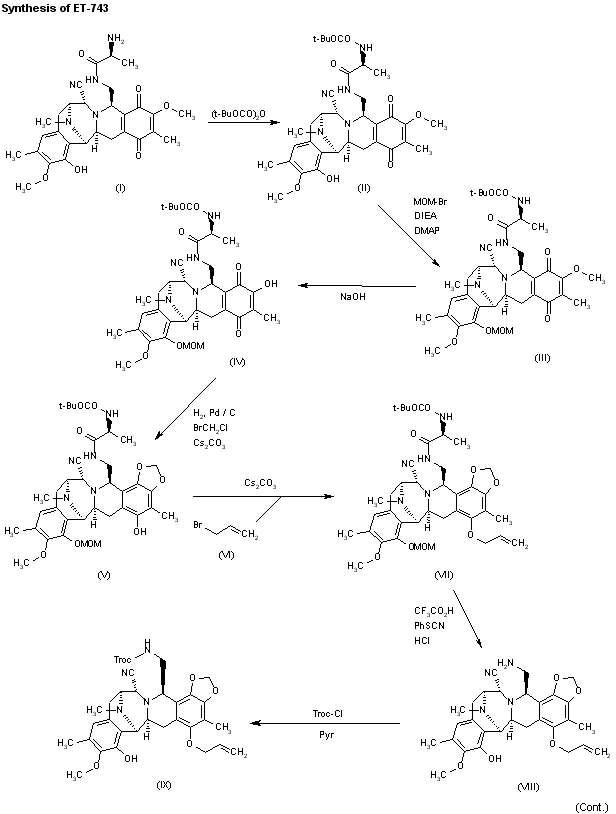
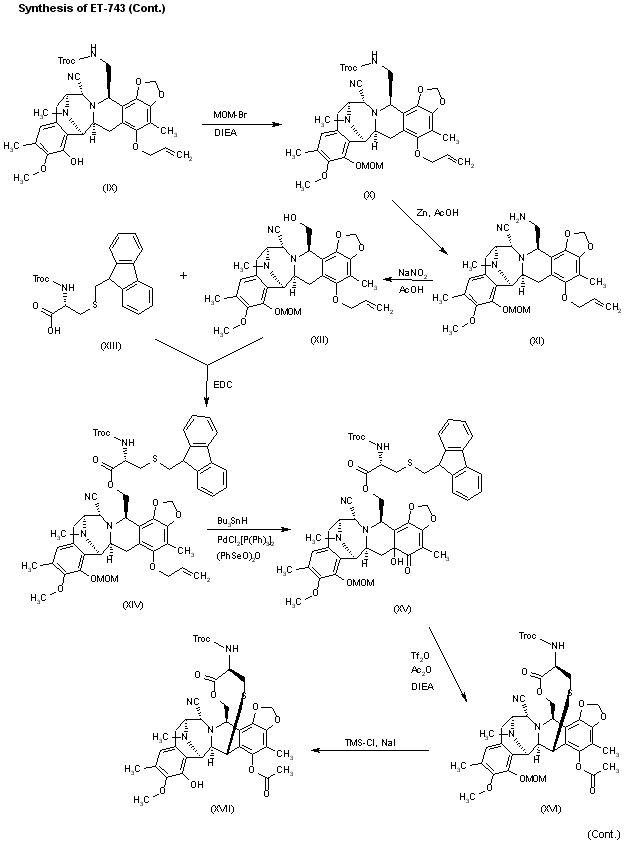
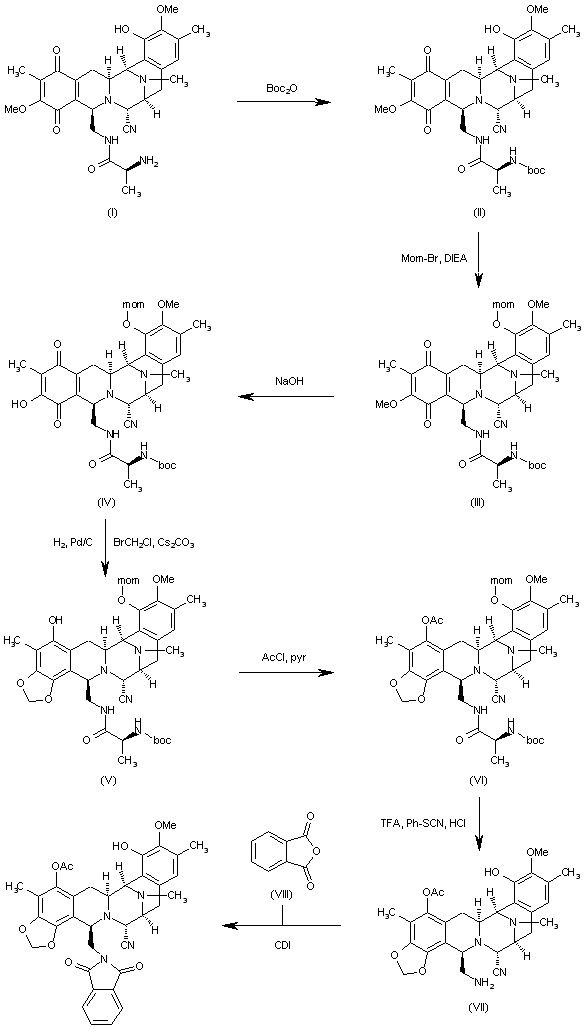
Reaction of cyanosafracin B (I) with Boc2O in ethanol gives the amino-protected compound (II), which is treated with methoxymethyl bromide (MOM-Br), DIEA and DMAP in acetonitrile yielding the O-protected compound (III). The demethylation of (III) with NaOH in methanol affords the hydroxyquinone (IV), which is reduced with H2 over Pd/C and cyclized with bromochloromethane and Cs2CO3 in hot DMF to provide compound (V). Reaction of (V) with allyl bromide (VI) and Cs2CO3 in DMF gives the allyl ether (VII), which first is treated with TFA, phenyl isothiocyanate and HCl to yield the primary amine (VIII) and then protected at the free NH2 group with Troc-Cl and pyridine, to afford the amino protected compound (IX).Org Lett 2000,2(16),2545
……………………………….
Reaction of (IX) with MOM-Br and DIEA as before affords the ether (X), which is treated with Zn/HOAc in order to regenerate the primary amino group giving (XI). The reaction of (XI) with NaNO2 and HOAc eliminates the NH2 group, affording the primary alcohol (XII), which is esterified with the protected (S)-cysteine (XIII) by means of EDC and DMAP in dichloromethane furnishing the cysteine ester (XIV). Reaction of (XIV) with Bu3SnH and PdCl2(PPh3)2, followed by oxidation with (PhSeO)2O in dichloromethane gives the hydroxyketone (XV), which is cyclized with Tf2O and Ac2O yielding the heptacyclic compound (XVI). Elimination of the MOM protecting group with TMSCl and NaI in CH3CN/CH2Cl2 affords the phenolic compound (XVII).
…………………….
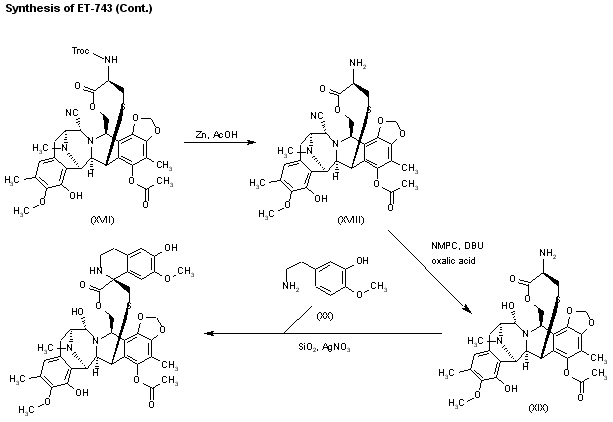
Intermediate (XVII) by a treatment with Zn and HOAc eliminates the Troc protecting group, giving the primary amine (XVIII). This compound by treatment with 4-formyl-1-methylpyridinium iodide (NMPC), DBU and oxalic acid in order to convert the nitrile group into an alcohol, provides compund (XIX), which is finally cyclized with 2-(3-hydroxy-4-methoxyphenyl)ethylamine (XX) by means of SiO2 / EtOH, followed treatment with and AgNO3 in acetonitrile/water.
……………………….
The reaction of cyanosafracin B (I) with Boc2O in ethanol gives the amino protected compound (II), which is treated with Mom-Br, DIEA and DMAP in acetonitrile yielding the O-protected compound (III). The demethylation of (III) with NaOH in methanol affords the hydroxyquinone (IV), which is reduced with H2 over Pd/C and cyclized with bromochloromethane and Cs2CO3 in hot DMF providing the methylenedioxy compound (V). The reaction of (V) with acetyl chloride and pyridine in dichloromethane gives the acetate (VI), which is treated with TFA, phenyl isothiocyanate and HCl yielding the primary amine (VII). Finally, this compound is treated with phthalic anhydride (VIII) and CDI in dichloromethane to afford the target phthalimide (phthalascidin Pt-650)
………………………………
Org. Lett., 2000, 2 (7), pp 993–996
DOI: 10.1021/ol0056729
Org. Lett., 2000, 2 (7), pp 993–996
DOI: 10.1021/ol0056729
…………………………
Enantioselective Total Synthesis of Ecteinascidin 743
Department of Chemistry, Harvard University Cambridge, Massachusetts 02138
J. Am. Chem. Soc., 1996, 118 (38), pp 9202–9203
DOI: 10.1021/ja962480t
……………………………….
Ecteinascidins are a group of marine alkaloid having antineoplasticity which is isolated from the extracted products from the marine tunicate habitat of the Caribbean sea by a very small amount. Arming the ecteinascidins, Et 743 has a very strong antineoplastic activity, studies to put it into practical use as a carcinostatic agent are limited, and the phase II clinical tests are now being carried out in ten countries in Europe and America. It is known that Et 743 has an effect of depressing the proliferation of cancer cells by 10 to 100 times more potent than (IC50=0.1-1 nM) Toxol, Camptotesin, Adriamycin or Mitomycin which are currently used carcinostatic agents.
From the background mentioned above, various studies for synthesis were carried out; however, the complete synthesis was only reported by Prof. E. J. Corey of Harvard University in the U.S.A. (J. Am. Chem. Soc. 1996, 118, 9202-9203, reference document A).
In the process of the total synthesis disclosed in Document A (refer to page 9202), the main feature of the process is that Et 743 is synthesized from the analogous compound to the compound represented by general formula 1 of the present invention via intermediates 4 and 8. That is, according to said process, the C4 site of ring B (regarding the location of rings, and the sites of atoms comprising the 6 membered ring, refer to general formula 1), which composes a 6 membered ring, is formed from the intermediate 4 at the first step. Since the atom C4 composing the ring B of the 6-membered ring H, which lacks reactivity, is bonded, it becomes necessary to perform an oxidation reaction at the C4 site on the B ring. This oxidation reaction is not effective and is carried out under harsh conditions; therefore production on an industrial scale is difficult, and also the yield is not good. Further, since the atom N12 site of the synthesized intermediate is substituted by an alkyl group which lacks reactivity, in this case substituted by a methyl group, it is not suited to the synthesis of various compounds. Although total synthesis was reported, the supplying source of Et 743 still depends on the natural sample whose supply is very scarce. Therefore, the establishment of the method for a large scale production of Et 743 is desired and requires accomplishing an effective synthesizing process.
Since ET 743 is known as a medicine having high antineoplasticity, and phthalascidin induced from the intermediate product at the synthesis of Et 743 displays the same activity to ET 743, the establishment of an effective and mild method for synthesis of ET 743 and analogous compounds thereof is strongly desired.
Therefore, the subject of the present invention is to accomplish the effective method for total synthesis of Et 743, and further, to provide not only Et 743 but also analogous compounds.
To dissolve the subject, the present invention uses retrosynthetic analysis for easy synthesis. It will be possible to form a B ring by a ring forming reaction at the ortho position of phenol, which binds an A ring to inner molecular aldehyde in a compound generated by the 4-8 reaction. Further, the present invention contemplates that the generated compound by the 4-8 reaction can be synthesized based on the polycondensation reaction of general formula 4, and general formula 5 via a compound of general formula 3. Then the total synthesis of Et 743, which is the aimed compound, can be accomplished by way of the compounds represented by general formulae 5, 4, 3, 2 and 1 and the specific structure of general formulae 1 and 2. This synthetic route provides for the analogous compounds of Et 743.





Mechanism of action
The biological
mechanism of action is believed to involve the production of
superoxide near the DNA strand, resulting in DNA backbone cleavage and cell
apoptosis. The actual mechanism is not yet known, but is believed to proceed from reduction of molecular oxygen into superoxide via an unusual auto-redox reaction on a hydroxyquinone moiety of the compound following. There is also some speculation the compound becomes ‘activated’ into its reactive
oxazolidine form.
Schematic of the unique and complex mode of action of trabectedin. The antitumor effects of trabectedin are due to multiple mechanisms involving DNA binding in the minor groove, interactions with DNA repair mechanisms, modulation of transcription regulation, and induction of microenvironment changes.
References
TRABECTEDIN
 |
| SYSTEMATIC (IUPAC) NAME |
|---|
| (1′R,6R,6aR,7R,13S,14S,16R)-6′,8,14-trihydroxy-7′,9-dimethoxy-4,10,23-trimethyl-19-oxo-3′,4′,6,7,12,13,14,16-octahydrospiro[6,16-(epithiopropano-oxymethano)-7,13-imino-6aH-1,3-dioxolo[7,8]isoquino[3,2-b][3]benzazocine-20,1′(2′H)-isoquinolin]-5-yl acetate |
| CLINICAL DATA |
|---|
| AHFS/DRUGS.COM | International Drug Names |
|---|
| LICENCE DATA | EMA:Link |
|---|
| LEGAL STATUS |
|
|---|
| ROUTES | Intravenous |
|---|
| PHARMACOKINETIC DATA |
|---|
| BIOAVAILABILITY | Not applicable (IV only) |
|---|
| PROTEIN BINDING | 94 to 98% |
|---|
| METABOLISM | Hepatic (mostly CYP3A4-mediated) |
|---|
| HALF-LIFE | 180 hours (mean) |
|---|
| EXCRETION | Mostly fecal |
|---|
| IDENTIFIERS |
|---|
| CAS NUMBER | 114899-77-3 |
|---|
| ATC CODE | L01CX01 |
|---|
| PUBCHEM | CID 108150 |
|---|
| IUPHAR LIGAND | 2774 |
|---|
| DRUGBANK | DB05109 |
|---|
| CHEMSPIDER | 16736970  |
|---|
| UNII | ID0YZQ2TCP  |
|---|
| |
|---|
| |
|---|
| CHEMICAL DATA |
|---|
| FORMULA | C39H43N3O11S |
|---|
| MOL. MASS | 761.84 g/mol |
|---|
……..
|
| 2 | * | Endo, “Synthetic Study on Ecteinascidin 743 Starting From D-Glucose“, Synlett 1999, No. 7, 1103-1105. |
| 3 | * | Endo, “Total Synthesis of Ecteinascidin 743“, J. Am. Chem. Soc. 2002, vol. 124, 6552-6554. |
| 4 | * | Hinterding, “Synthesis and In Vitro Evaluation of the Ras Farnesyltransferase Inhibitor Pepticinnamin E“, Angew. Chem. Int. Ed. 1998, 37, No. 9 1236-1239. |
| 5 | * | Tohma, “Synthesis of Optically Active alpha-Arylglycines: Stereoselective Mannich-Type Reaction with a New Chiral Template“, Synlett 2001, No. 7, 1179-1181.Hamprecht, D.W.; Berge, J.M.; Copley, R.C.B.; Eggleston, D.S.; Houge-Frydrych, C.S.V.; Jarvest, R.L.; Mensah, L.M.; O’Hanlon, P.J.; Pope, A.J.; Rittenhouse, S.
Derivatives of the natural product SB-219383 and synthetic analogues: Potent inhibitors of bacterial tyrosyl tRNA synthetase
16th Int Symp Med Chem (September 18-22, Bologna) 2000, Abst PA-155Cuevas, C.; Perez, M.; Martin, M.J.; et al.
Synthesis of ecteinascidin ET-743 and phathalascidin Pt-650 from cyanosafracin B
Org Lett 2000, 2(16): 2545
|
| PATENT | SUBMITTED | GRANTED |
|---|
| Assay for identifying biological targets of polynucleotide-binding compounds [US2008096201] | 2008-04-24 | |
| Compounds of the saframycin-ecteinascidin series, uses, and synthesis thereof [US6936714] | 2004-07-01 | 2005-08-30 |
| Method For Total Synthesis Of Ecteinascidins And Intermediate Compounds Thereof [US7807833] | 2009-08-06 | 2010-10-05 |
| Method For Total Synthesis Of Ecteinascidins And Intermediate Compounds Thereof [US7820838] | 2009-02-05 | 2010-10-26 |
| Assay for identifying biological targets of polynucleotide-binding compounds [US7183054] | 2004-12-09 | 2007-02-27 |