
Saffron (pronounced /ˈsæfrən/ or /ˈsæfrɒn/)[1] is a spice derived from the flower of Crocus sativus, commonly known as the saffron crocus. Crocus is a genus in the family Iridaceae. Saffron crocus grows to 20–30 cm (8–12 in) and bears up to four flowers, each with three vivid crimson stigmas, which are the distal end of a carpel.[2] The styles and stigmas are collected and dried to be used as a seasoning and colouring agent in cooking. Saffron, long among the world's most costly spices by weight,[3][4][5] is native to Greece orSouthwest Asia[6][4] and was first cultivated in Greece.[7] As a genetically monomorphic clone,[8] it was slowly propagated throughout much of Eurasia and was later brought to parts of North Africa, North America, and Oceania. The saffron crocus, unknown in the wild, probably descends from Crocus cartwrightianus, which originated in Crete;[8] C. thomasii and C. pallasii are other possible precursors.[9][10] The saffron crocus is a triploid that is "self-incompatible" and male sterile; it undergoes aberrant meiosis and is hence incapable of independent sexual reproduction—all propagation is by vegetative multiplication via manual "divide-and-set" of a starter clone or by interspecific hybridisation.[11][10] If C. sativus is a mutant form of C. cartwrightianus, then it may have emerged via plant breeding, which would have selected for elongated stigmas, in late Bronze Age Crete.[12] Saffron's taste and iodoform- or hay-like fragrance result from the chemicals picrocrocin and safranal.[13][14] It also contains a carotenoid pigment, crocin, which imparts a richgolden-yellow hue to dishes and textiles. Its recorded history is attested in a 7th-century BC Assyrian botanical treatise compiled under Ashurbanipal,[15] and it has been traded and used for over four millennia. Iran now accounts for approximately 90% of the world production of saffron.[16] Saffron is obtained from dried style and stigma of reddish-orange flowers of a plant. Kesar or Saffron is the most expensive spice of world as stigmas of about 60, 000 hand collected flowers provide only half- kilograms of it. Saffron is used as coloring and flavoring ingredient in the preparation of various dishes. It is also used as traditional medicine for many diseases and in cosmetics. Saffron has a distinct aromatic odour and a bitter, pungent taste. Medicinally it is stimulant (stimulates levels of physiological or nervous activity), aphrodisiac, improves digestion and appetite. It increases blood flow in pelvic region on oral intake. Its over-doses is a narcotic poison. Saffron is always used in small doses. It is a popular remedy for promoting menstruation.

- The plant is grown in India, Spain, France, Italy, the Middle East, and the eastern Mediterranean region.
- Over 200,000 crocus stigmas must be harvested to produce one pound of saffron.
- Saffron is harvested by drying the orange stigma which are 3 of them in one Crocus sativus flower over fire.
- This volume makes the herb extremely expensive and quite often adulterated.
- Saffron is prescribed as a herbal remedy to stimulate the digestive system, ease colic and stomach discomfort, and minimize gas.
- It is also used as an emmenagogue, to stimulate and promote menstrual flow in women.
- Additional human studies have indicated that saffron has powerful antioxidant properties; that is, it helps to protect living tissues from free radicals and other harmful effects of oxidation.
- Two chemical components of saffron extract, crocetin and crocin, reportedly improved memory and learning skills. These properties indicate that saffron extract may be a useful treatment for neurodegenerative disorders and related memory impairment.
- In ancient India, robes were traditionally dyed a golden color from the crocin chemical dye that is found in saffron.
- In fact, after Buddha had died, the Buddhist priests made this golden saffron color their official color of their robes.
- Saffron was used by Greeks and Romans as a perfume on behalf of its pleasant aroma.
- Cleopatra used to use saffron as a type of cosmetic. And now a days it is used in face creams as a fairness cream.
- In the Middle Ages, one could be sentenced to the punishment of being buried alive if they tried to alter saffron by adding in other substances.
- Romans used to take baths infused with saffron.
- In order to cure hang-overs, Romans would sleep with expensive pillows that were stuffed with saffron.
- Saffron is extensively used in Indian Cuisine and Middle Eastern Cuisine.
Scientific classification
- Kingdom:Plantae
- Division:Magnoliophyta
- Class:Liliopsida
- Order:Asparagales
- Family:Iridaceae
- Genus:Crocus
- Species:C. sativus
Vernacular Names
SANSKRIT:Bhavarakta, Saurab, Mangalya, Kumkum ENGLISH:Saffron, Crocus PERSIAN:Zafrahn;Zipharana;GUJARATI:Keshar, Kesar KANNADA:Kunkuma, Kesari, MALAYALAM:Kunkuma Puvu MARATHI:Keshar PUNJABI:Kesar, Keshar TAMIL:Kungumapuvu TELUGU:Kunkuma Puvvu URDU:Zafran Parts Used:Dried stigmas and tops of the styles of Crocus sativus flowers. Habitat:Saffron is Cultivated in Kashmir, Kishtwar (Jammu) and in Nepal. Commercially, it is grown in Spain, France, Italy, Greece, Turkey, and China. Energetics:Pungent, bitter, Hot in potency
Plant description
Perennial tuber plant;Leaves radical, linear, dark green above, pale green below, enclosed in a membranous sheath;large Apurple or lilac colored flowers;Corolla in two segments, between which the long styles hang out;Stigmas three, large, nearly an inch long, rolled at the edges, bright orange bitter and warming taste.
Constituents of Saffron
Saffron contains three crystalline colouring matters ?-crocetin, ?-crocetin and ?-crocetin. It also contains essential oil a number of carotenoid pigments. The essential oil obtained from stigmas contains thirty-four or more components, viz. terpenes, terpene alcohols, and esters.
Medicinal Uses of Saffron
Saffron is used as condiment and colouring ingredient in several dishes. It is also used as a medicinal herb in fevers, enlargement of the liver, cough and asthma, anaemia, seminal debility rheumatism and neuralgia. Saffron is nervine tonic, sedative, antispasmodic expectorant, stomachic, diaphoretic and emmenagogue. In low doses Saffron stimulates gastric secretion and thus improves digestion. In large dose it increases flow of blood in pelvic region, stimulate uterine smooth muscles and can cause abortion.
- Saffron oral use gives relief in respiratory ailments. In cough and cold a pinch of Saffron is taken with a glass of milk.
- In painful urination and other urinary disorder the decoction of Saffron or infused tea should be taken.
- In irritation in eyes, crushed saffron should be mixed with honey and this should be applied in eyes.
- In looseness of bowels saffron is given children with ghee. It can also be given with half a teaspoon of lemon juice.
- For pneumonia in kids, few threads of saffron are added to 10-15 ml juice of bitter gourd leaves and given twice a day.
- Saffron is added to meals for regulating the menstrual cycle. It also gives relief in painful menstruation, PMS (premenstrual syndrome) and promotes fertility.
- For sexual weakness, about 250 mg of saffron is taken with milk twice a day for one week.
- Saffron improves digestion and appetite.
- To get relief from dry cough one should drink one hot glass of milk added with turmeric, and few strands of saffron.
- Saffron in paste form is applied topically for head-ache.
- Its external application is also useful in sores, bruises and skin diseases. It is applied on face for improving complexion and treating hyper-pigmented spots.
- It is also used for patchy loss of hair. For this purpose a paste of liquorice (mulethi) made by grinding the pieces in milk with a pinch of saffron is applied over the bald patches in the night before going to bed.
- A famous Ayurvedic preparation containing Kesar or saffron is kumkumadi tailam. This medicated saffron/kumkum oil is applied on pimples marks, dark spots, dark circles, wrinkles etc.
The recommended doses of Saffron below one gram. Toxic dose is 1.5g–5 g.
Etymology
Further information: History of saffron
A degree of uncertainty surrounds the origin of the English word, "saffron" although it can be traced to have stemmed immediately from 12th-century Old French term safran, which comes from the Latin word safranum. Safranum comes from the Persian intercessor زعفران, or za'ferân. Old Persian is the first language in which the use of saffron in cooking is recorded, with references dating back thousands of years.
Species
Main article: Crocus sativus
Description
The domesticated saffron crocus, Crocus sativus, is an autumn-flowering perennial plant unknown in the wild. Its progenitors are possibly the eastern Mediterranean autumn-flowering Crocus cartwrightianus,[17][10] which is also known as "wild saffron"[18] and originated in Greece.[14] The saffron crocus probably resulted when C. cartwrightianus was subjected to extensive artificial selection by growers seeking longer stigmas. C. thomasii and C. pallasii are other possible sources.[9][10] It is a sterile triploid form, which means that three homologous sets of chromosomes compose each specimen's genetic complement; C. sativus bears eight chromosomal bodies per set, making for 24 in total.[2] Being sterile, the purple flowers of C. sativus fail to produce viable seeds; reproduction hinges on human assistance: clusters of corms, underground, bulb-like, starch-storing organs, must be dug up, divided, and replanted. A corm survives for one season, producing via this vegetative division up to ten "cormlets" that can grow into new plants in the next season.[17] The compact corms are small, brown globules that can measure as large as 5 cm (2.0 in) in diameter, have a flat base, and are shrouded in a dense mat of parallel fibres; this coat is referred to as the "corm tunic". Corms also bear vertical fibres, thin and net-like, that grow up to 5 cm above the plant's neck.[2]
The plant grows to a height of 20–30 cm (8–12 in), and sprouts 5–11 white and non-photosynthetic leaves known ascataphylls. These membrane-like structures cover and protect the crocus's 5 to 11 true leaves as they bud and develop. The latter are thin, straight, and blade-like green foliage leaves, which are 1–3 mm in diameter, either expand after the flowers have opened ("hysteranthous") or do so simultaneously with their blooming ("synanthous").C. sativus cataphylls are suspected by some to manifest prior to blooming when the plant is irrigated relatively early in the growing season. Its floral axes, or flower-bearing structures, bear bracteoles, or specialised leaves that sprout from the flower stems; the latter are known as pedicels.[2] After aestivating in spring, the plant sends up its true leaves, each up to 40 cm (16 in) in length. In autumn, purple buds appear. Only in October, after most other flowering plants have released their seeds, do its brilliantly hued flowers develop; they range from a light pastel shade of lilac to a darker and more striated mauve.[19] The flowers possess a sweet, honey-like fragrance. Upon flowering, plants average less than 30 cm (12 in) in height.[20] A three-pronged style emerges from each flower. Each prong terminates with a vivid crimson stigma 25–30 mm (0.98–1.18 in) in length.[17]
Cultivation
Crocus sativus thrives in the Mediterranean maquis, an ecotype superficially resembling the North American chaparral, and similar climates where hot and dry summer breezes sweep semi-arid lands. It can nonetheless survive cold winters, tolerating frosts as low as −10 °C (14 °F) and short periods of snow cover.[17][21] Irrigation is required if grown outside of moist environments such as Kashmir, where annual rainfall averages 1,000–1,500 mm (39–59 in); saffron-growing regions in Greece (500 mm or 20 in annually) and Spain (400 mm or 16 in) are far drier than the main cultivating Iranian regions. What makes this possible is the timing of the local wet seasons; generous spring rains and drier summers are optimal. Rain immediately preceding flowering boosts saffron yields; rainy or cold weather during flowering promotes disease and reduces yields. Persistently damp and hot conditions harm the crops,[22] and rabbits, rats, and birds cause damage by digging up corms. Nematodes, leaf rusts, and corm rot pose other threats. Yet Bacillus subtilis inoculation may provide some benefit to growers by speeding corm growth and increasing stigma biomass yield.[23]
The plants fare poorly in shady conditions; they grow best in full sunlight. Fields that slope towards the sunlight are optimal (i.e., south-sloping in the Northern Hemisphere). Planting is mostly done in June in the Northern Hemisphere, where corms are lodged 7–15 cm (2.8–5.9 in) deep; its roots, stems, and leaves can develop between October and February.[2] Planting depth and corm spacing, in concert with climate, are critical factors in determining yields. Mother corms planted deeper yield higher-quality saffron, though form fewer flower buds and daughter corms. Italian growers optimise thread yield by planting 15 cm (5.9 in) deep and in rows 2–3 cm (0.79–1.18 in) apart; depths of 8–10 cm (3.1–3.9 in) optimise flower and corm production. Greek, Moroccan, and Spanish growers employ distinct depths and spacings that suit their locales. C. sativus prefers friable, loose, low-density, well-watered, and well-drained clay-calcareous soils with high organic content. Traditional raised beds promote good drainage. Soil organic content was historically boosted via application of some 20–30 tonnes of manure per hectare. Afterwards, and with no further manure application, corms were planted.[24] After a period of dormancy through the summer, the corms send up their narrow leaves and begin to bud in early autumn. Only in mid-autumn do they flower. Harvests are by necessity a speedy affair: after blossoming at dawn, flowers quickly wilt as the day passes.[25] All plants bloom within a window of one or two weeks.[26]Roughly 150 flowers together yield 1 g (0.035 oz) of dry saffron threads; to produce 12 g (0.42 oz) of dried saffron (or 72 g (2.5 oz) moist and freshly harvested), 1 kg (2.2 lb) of flowers are needed; 1 lb (0.45 kg) yields 0.2 oz (5.7 g) of dried saffron. One freshly picked flower yields an average 30 mg (0.0011 oz) of fresh saffron or 7 mg (0.00025 oz) dried.[24]
Spice
Chemistry

safranal moiety
|
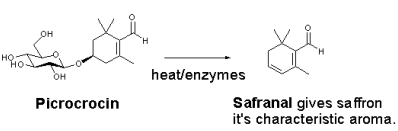 Picrocrocin is a monoterpene glycoside precursor of safranal. It is found in the spice saffron, which comes from the crocus flower.Picrocrocin has a bitter taste, and is the chemical most responsible for the taste of saffron. During the drying process, picrocrocin liberates the aglycone (HTCC, C10H16O2) due to the action of the enzyme glucosidase. The aglycone is then transformed to safranal by dehydration. Picrocrocin is a degradation product of the carotenoidzeaxanthin. Caballero-Ortega H, Pereda-Miranda R, Abdullaev FI (2007). "HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources". Food Chemistry 100 (3): 1126–1131. doi:10.1016/j.foodchem.2005.11.020.
Picrocrocin is a monoterpene glycoside precursor of safranal. It is found in the spice saffron, which comes from the crocus flower.Picrocrocin has a bitter taste, and is the chemical most responsible for the taste of saffron. During the drying process, picrocrocin liberates the aglycone (HTCC, C10H16O2) due to the action of the enzyme glucosidase. The aglycone is then transformed to safranal by dehydration. Picrocrocin is a degradation product of the carotenoidzeaxanthin. Caballero-Ortega H, Pereda-Miranda R, Abdullaev FI (2007). "HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources". Food Chemistry 100 (3): 1126–1131. doi:10.1016/j.foodchem.2005.11.020.- Pfander, H.; Schurtenberger, H.; Biosynthesis of C20-carotenoids in Crocus sativus. Phytochemistry 1982, 21, 1039-1042. http://dx.doi.org/10.1016/S0031-9422(00)82412-7
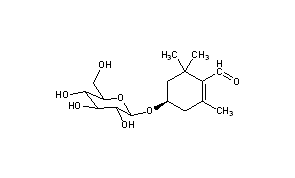
Picrocrocin
CAS Registry Number: 138-55-6
CAS Name: (4R)-4-(b-D-Glucopyranosyloxy)-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde
Additional Names: saffron-bitter
Molecular Formula: C16H26O7
Molecular Weight: 330.37
Percent Composition: C 58.17%, H 7.93%, O 33.90%
Literature References: From stigmas of Crocus sativus L., Iridaceae. Isoln: Kayser, Ber. 17, 2228 (1884). Structure: Kuhn, Winterstein, Ber. 67, 344 (1934). Exerts sex-determining influences in the plant organism: Kuhn, Angew. Chem. 53, 1 (1940). Its moieties are glucose and safranal, q.q.v. Abs config: Buchecker, Eugster, Helv. Chim. Acta 56, 1121 (1973). Synthesis: H. Mayer, J.-M. Santer, Helv. Chim. Acta 63, 1463 (1980).
Properties: Crystals, mp 154-156°. [a]D20 -58° (c = 0.6). Bitter taste. Alkali unstable. Sol in water, alcohol; slightly sol in chloroform, ether. Practically insol in petr ether, benzene.
Melting point: mp 154-156°
Optical Rotation: [a]D20 -58° (c = 0.6)

| Picrocrocin |
| 1H NMR spectrum | Predict NMR spectrum (requires installed Java plugin) Predict NMR spectrum (requires HTML5 compatible browser) |
| 13C NMR spectrum | Predict NMR spectrum (requires HTML5 compatible browser) |
 | |
| NAMES | |
|---|---|
| IUPAC names
4-(β-D-glucopyranosyloxy)- 2,6,6-trimethyl-1-cyclohexene- 1-carboxaldehyde
| |
| IDENTIFIERS | |
| CAS number | 138-55-6 |
| ChemSpider | 115678 |
| Jmol-3D images | Image (138-55-6) |
| PubChem | 130796 |
| PROPERTIES | |
| C16H26O7 | |
| Molar mass | 330.37 g/mol |
| Density | 1.31 g/mL |
| Melting point | 154 to 156 °C (309 to 313 °F; 427 to 429 K) |
| Boiling point | 520.4 °C (968.7 °F; 793.5 K) |
Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa)
| |
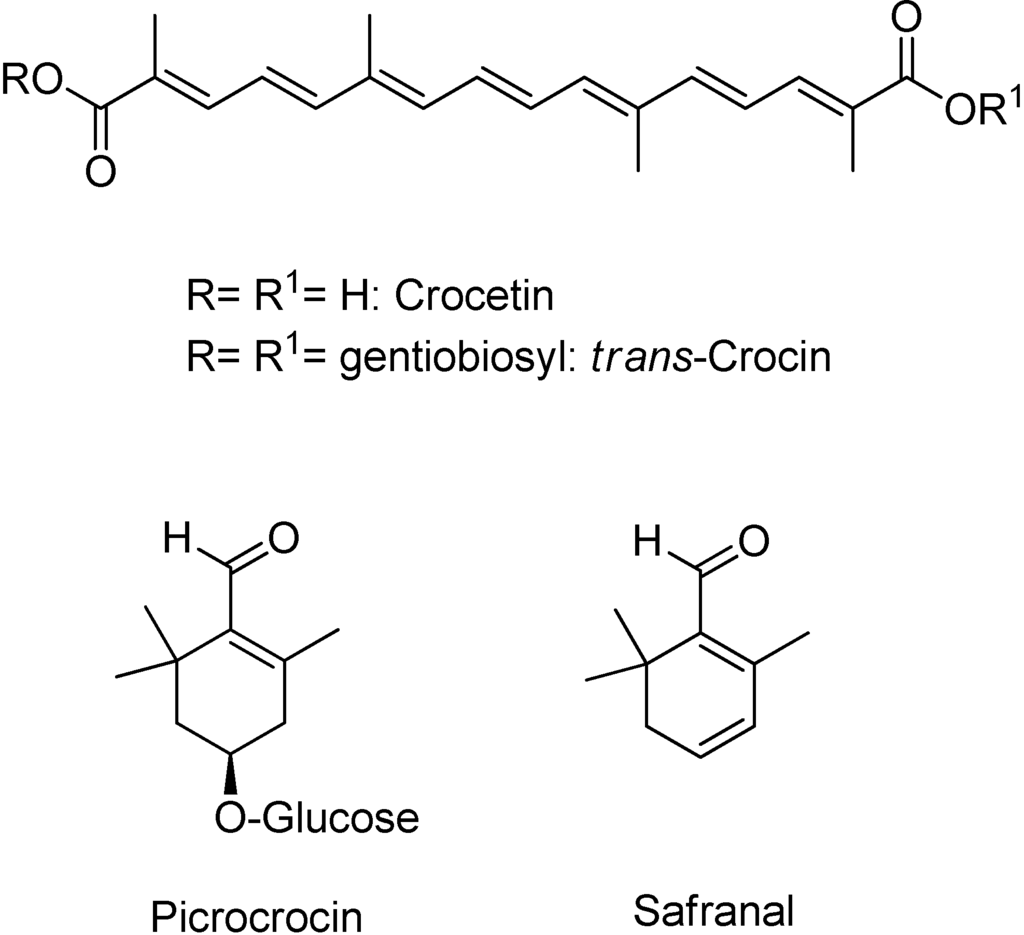
Saffron contains more than 150 volatile and aroma-yielding compounds. It also has many nonvolatile active components,[28] many of which are carotenoids, including zeaxanthin, lycopene, and various α- and β-carotenes. However, saffron's golden yellow-orange colour is primarily the result of α-crocin. This crocin is trans-crocetin di-(β-D-gentiobiosyl) ester; it bears the systematic (IUPAC) name 8,8-diapo-8,8-carotenoic acid. This means that the crocin underlying saffron's aroma is a digentiobiose ester of the carotenoid crocetin.[28] Crocins themselves are a series ofhydrophilic carotenoids that are either monoglycosyl or diglycosyl polyene esters of crocetin.[28] Crocetin is a conjugated polyene dicarboxylic acidthat is hydrophobic, and thus oil-soluble. When crocetin is esterified with two water-soluble gentiobioses, which are sugars, a product results that is itself water-soluble. The resultant α-crocin is a carotenoid pigment that may comprise more than 10% of dry saffron's mass. The two esterified gentiobioses make α-crocin ideal for colouring water-based and non-fatty foods such as rice dishes.[7]

The bitter glucoside picrocrocin is responsible for saffron's flavour. Picrocrocin (chemical formula:C 16H 26O 7; systematic name: 4-(β-D-glucopyranosyloxy)-2,6,6- trimethylcyclohex-1-ene-1-carboxaldehyde) is a union of an aldehyde sub-element known as safranal (systematic name: 2,6,6-trimethylcyclohexa-1,3-diene-1-carboxaldehyde) and a carbohydrate. It has insecticidal and pesticidal properties, and may comprise up to 4% of dry saffron. Picrocrocin is a truncated version of the carotenoidzeaxanthin that is produced via oxidative cleavage, and is the glycoside of the terpene aldehyde safranal. The reddish-coloured zeaxanthin is, incidentally, one of the carotenoids naturally present within the retina of the human eye.[29] When saffron is dried after its harvest, the heat, combined with enzymatic action, splits picrocrocin to yield D–glucose and a free safranal molecule.[27] Safranal, a volatile oil, gives saffron much of its distinctive aroma.[13][30] Safranal is less bitter than picrocrocin and may comprise up to 70% of dry saffron's volatile fraction in some samples.[29] A second element underlying saffron's aroma is 2-hydroxy-4,4,6-trimethyl-2,5-cyclohexadien-1-one, which produces a scent described as saffron, dried hay-like.[31] Chemists find this is the most powerful contributor to saffron's fragrance, despite its presence in a lesser quantity than safranal.[31] Dry saffron is highly sensitive to fluctuating pH levels, and rapidly breaks down chemically in the presence of light andoxidising agents. It must, therefore, be stored away in air-tight containers to minimise contact with atmospheric oxygen. Saffron is somewhat more resistant to heat.
Grades and ISO 3632 categories[edit]
Saffron is not all of the same quality and strength. Strength is related to several factors including the amount of style picked along with the red stigma. Age of the saffron is also a factor. More style included means the saffron is less strong gram for gram, because the colour and flavour are concentrated in the red stigmas. Saffron from Iran, Spain and Kashmir is classified into various grades according to the relative amounts of red stigma and yellow styles it contains. Grades of Iranian saffron are: "sargol" (red stigma tips only, strongest grade), "pushal" or "pushali" (red stigmas plus some yellow style, lower strength), "bunch" saffron (red stigmas plus large amount of yellow style, presented in a tiny bundle like a miniature wheatsheaf) and "konge" (yellow style only, claimed to have aroma but with very little, if any, colouring potential). Grades of Spanish saffron are "coupé" (the strongest grade, like Iranian sargol), "mancha" (like Iranian pushal), and in order of further decreasing strength "rio", "standard" and "sierra" saffron. The word "mancha" in the Spanish classification can have two meanings: a general grade of saffron or a very high quality Spanish-grown saffron from a specific geographical origin. Real Spanish-grown La Mancha saffron has PDO protected status and this is displayed on the product packaging. Spanish growers fought hard for Protected Status because they felt that imports of Iranian saffron re-packaged in Spain and sold as "Spanish Mancha saffron" were undermining the genuine La Mancha brand. Countries producing less saffron do not have specialised words for different grades and may only produce one grade. Artisan producers in Europe and New Zealand have offset their higher labour charges for saffron harvesting by targeting quality, only offering extremely high grade saffron. In addition to descriptions based on how the saffron is picked, saffron may be categorised under the international standard ISO 3632 after laboratory measurement of crocin (responsible for saffron's colour), picrocrocin (taste), and safranal (fragrance or aroma) content.[32] However, often there is no clear grading information on the product packaging and little of the saffron readily available in UK is labelled with ISO category. This lack of information makes it hard for customers to make informed choices when comparing prices and buying saffron. Under ISO 3632, determination of non-stigma content ("floral waste content") and other extraneous matter such as inorganic material ("ash") are also key. Grading standards are set by the International Organization for Standardization, a federation of national standards bodies. ISO 3632 deals exclusively with saffron and establishes three categories: III (poorest quality), II, and I (finest quality). Formerly there was also category IV, which was below category III. Samples are assigned categories by gauging the spice's crocin and picrocrocin content, revealed by measurements of specific spectrophotometric absorbance. Safranal is treated slightly differently and rather than there being threshold levels for each category, samples must give a reading of 20-50 for all categories. These data are measured through spectrophotometry reports at certified testing laboratories worldwide. Higher absorbances imply greater levels of crocin, picrocrocin and safranal, and thus a greater colouring potential and therefore strength per gram. The absorbance reading of crocin is known as the "colouring strength" of that saffron. Saffron's colouring strength can range from lower than 80 (for all category IV saffron) up to 200 or greater (for category I). The world's finest samples (the selected, most red-maroon, tips of stigmas picked from the finest flowers) receive colouring strengths in excess of 250, making such saffron over three times more powerful than category IV saffron. Market prices for saffron types follow directly from these ISO categories. Sargol and coupé saffron would typically fall into ISO 3632 category I. Pushal and mancha would probably be assigned to category II. On many saffron packaging labels, neither the ISO 3632 category nor the colouring strength (the measurement of crocin content) is displayed. However, many growers, traders, and consumers reject such lab test numbers. Some people prefer a more holistic method of sampling batches of threads for taste, aroma, pliability, and other traits in a fashion similar to that practised by experienced wine tasters.[33] However, ISO 3632 grade and colouring strength information allow consumers to make instant comparisons between the quality of different saffron brands, without needing to purchase and sample the saffron. In particular, consumers can work out value for money based on price per unit of colouring strength rather than price per gram, given the wide possible range of colouring strengths that different kinds of saffron can have. Despite attempts at quality control and standardisation, an extensive history of saffron adulteration, particularly among the cheapest grades, continues into modern times. Adulteration was first documented in Europe's Middle Ages, when those found selling adulterated saffron were executed under the Safranschou code.[34] Typical methods include mixing in extraneous substances like beets, pomegranate fibres, red-dyed silk fibres, or the saffron crocus's tasteless and odourless yellow stamens. Other methods included dousing saffron fibres with viscid substances like honey or vegetable oil to increase their weight. However, powdered saffron is more prone to adulteration, with turmeric, paprika, and other powders used as diluting fillers. Adulteration can also consist of selling mislabelled mixes of different saffron grades. Thus, in India, high-grade Kashmiri saffron is often sold and mixed with cheaper Iranian imports; these mixes are then marketed as pure Kashmiri saffron, a development that has cost Kashmiri growers much of their income.[35][36]
Types
The various saffron crocus cultivars give rise to thread types that are often regionally distributed and characteristically distinct. Varieties (not varieties in the botanical sense) from Spain, including the tradenames "Spanish Superior" and "Creme", are generally mellower in colour, flavour, and aroma; they are graded by government-imposed standards. Italian varieties are slightly more potent than Spanish. The most intense varieties tend to be Iranian. Various "boutique" crops are available from New Zealand, France, Switzerland, England, the United States, and other countries—some of them organically grown. In the U.S., Pennsylvania Dutch saffron—known for its "earthy" notes—is marketed in small quantities.[37][38] Consumers may regard certain cultivars as "premium" quality. The "Aquila" saffron, or zafferano dell'Aquila, is defined by high safranal and crocin content, distinctive thread shape, unusually pungent aroma, and intense colour; it is grown exclusively on eight hectares in the Navelli Valley of Italy's Abruzzo region, near L'Aquila. It was first introduced to Italy by a Dominican monk from Inquisition-era Spain. But the biggest saffron cultivation in Italy is in San Gavino Monreale, Sardinia, where it is grown on 40 hectares, representing 60% of Italian production; it too has unusually high crocin, picrocrocin, and safranal content. Another is the "Mongra" or "Lacha" saffron of Kashmir (Crocus sativus 'Cashmirianus'), which is among the most difficult for consumers to obtain. Repeated droughts, blights, and crop failures in the Indian-controlled areas of Kashmir combine with an Indian export ban to contribute to its prohibitive overseas prices. Kashmiri saffron is recognisable by its dark maroon-purple hue; it is among the world's darkest, which hints at strong flavour, aroma, and colouring effect.
History
Main article: History of saffron

The documented history of saffron cultivation spans more than three millennia.[17] The wild precursor of domesticated saffron crocus wasCrocus cartwrightianus. Human cultivators bred wild specimens by selecting for unusually long stigmas; thus, a sterile mutant form of C. cartwrightianus, C. sativus, likely emerged in late Bronze Age Crete.[12]
Eastern
Saffron was detailed in a 7th-century BC Assyrian botanical reference compiled under Ashurbanipal.[15]Documentation of saffron's use over the span of 4,000 years in the treatment of some 90 illnesses has been uncovered.[39] Saffron-based pigments have indeed been found in 50,000 year-old depictions of prehistoric places in northwest Iran.[40][41] The Sumerians later used wild-growing saffron in their remedies and magical potions.[42] Saffron was an article of long-distance trade before the Minoan palace culture's 2nd millennium BC peak. Ancient Persians cultivated Persian saffron (Crocus sativus 'Hausknechtii') in Derbena, Isfahan, and Khorasan by the 10th century BC. At such sites, saffron threads were woven into textiles,[40] ritually offered to divinities, and used in dyes, perfumes, medicines, and body washes.[43]Saffron threads would thus be scattered across beds and mixed into hot teas as a curative for bouts of melancholy. Non-Persians also feared the Persians' usage of saffron as a drugging agent and aphrodisiac.[44] During his Asian campaigns, Alexander the Great used Persian saffron in his infusions, rice, and baths as a curative for battle wounds. Alexander's troops imitated the practice from the Persians and brought saffron-bathing to Greece.[45] Conflicting theories explain saffron's arrival in South Asia. Kashmiri and Chinese accounts date its arrival anywhere between 2500–900 years ago.[46][47][48] Historians studying ancient Persian records date the arrival to sometime prior to 500 BC,[7] attributing it to a Persian transplantation of saffron corms to stock new gardens and parks.[49] Phoenicians then marketed Kashmiri saffron as a dye and a treatment for melancholy. Its use in foods and dyes subsequently spread throughout South Asia. Buddhist monks wear saffron-coloured robes; however, the robes are not dyed with costly saffron but turmeric, a less expensive dye, or jackfruit.[50] Monks' robes are dyed the same colour to show equality with each other, and turmeric or ochre were the cheapest, most readily available dyes. Gamboge is now used to dye the robes.[51] Some historians believe that saffron came to China with Mongol invaders from Persia.[52] Yet saffron is mentioned in ancient Chinese medical texts, including the forty-volume pharmacopoeia titled Shennong Bencaojing (神農本草經: "Shennong's Great Herbal", also known as Pen Ts'ao or Pun Tsao), a tome dating from 300–200 BC. Traditionally credited to the fabled Yan ("Fire") Emperor (炎帝) Shennong, it discusses 252 phytochemical-based medical treatments for various disorders.[53] Nevertheless, around the 3rd century AD, the Chinese were referring to saffron as having a Kashmiri provenance. According to Chinese herbalist Wan Zhen, "[t]he habitat of saffron is in Kashmir, where people grow it principally to offer it to the Buddha." Wan also reflected on how it was used in his time: "The flower withers after a few days, and then the saffron is obtained. It is valued for its uniform yellow colour. It can be used to aromatise wine."[48]
Wider Near East, Western Europe and the USA
The Minoans portrayed saffron in their palace frescoes by 1600–1500 BC; they hint at its possible use as a therapeutic drug.[39][54] Ancient Greek legends told of sea voyages to Cilicia, where adventurers sought what they believed were the world's most valuable threads.[21] Another legend tells of Crocus and Smilax, whereby Crocus is bewitched and transformed into the first saffron crocus.[40] Ancient perfumers in Egypt, physicians inGaza, townspeople in Rhodes,[55] and the Greek hetaerae courtesans used saffron in their scented waters, perfumes and potpourris, mascaras and ointments, divine offerings, and medical treatments.[44] In late Hellenistic Egypt, Cleopatra used saffron in her baths so that lovemaking would be more pleasurable.[56] Egyptian healers used saffron as a treatment for all varieties of gastrointestinal ailments.[57] Saffron was also used as a fabric dye in such Levantine cities as Sidon and Tyre inLebanon.[58] Aulus Cornelius Celsus prescribes saffron in medicines for wounds, cough, colic, and scabies, and in the mithridatium.[59] Such was the Romans' love of saffron that Roman colonists took it with them when they settled in southern Gaul, where it was extensively cultivated until Rome's fall. Competing theories state that saffron only returned to France with 8th-century AD Moors or with the Avignon papacy in the 14th century AD.[60] European saffron cultivation plummeted after the Roman Empire went into eclipse. As with France, the spread of Islamic civilisation may have helped reintroduce the crop to Spain and Italy.[61] The 14th-century Black Death caused demand for saffron-based medicaments to peak, and Europe imported large quantities of threads via Venetian and Genoan ships from southern and Mediterranean lands such as Rhodes. The theft of one such shipment by noblemen sparked the fourteen-week-long Saffron War.[62] The conflict and resulting fear of rampant saffron piracy spurred corm cultivation in Basel; it thereby grew prosperous.[63] The crop then spread to Nuremberg, where endemic and insalubrious adulteration brought on the Safranschou code—whereby culprits were variously fined, imprisoned, and executed.[64] Saffron cultivation was introduced into England in around 1350, the story being that corms were smuggled from the Levant in a special hollow compartment of a pilgrim's staff .[65]The crop seems to have been initially grown in monastic gardens for medicinal use, only being planted in the less kind conditions of open fields many decades later. Soil and climatic conditions meant that by the sixteenth century, saffron cultivation had centred on Eastern England. The Essex town of Saffron Walden, named for its new speciality crop, emerged as a prime saffron growing and trading centre. However, an important omission in a botanical book published in the 1790s meant that the true extent of saffron growing in the eastern counties has been long overlooked .[66] North Norfolk (especially the area around Walsingham), southern Cambridgeshire and a small area of west Suffolk also produced saffron. Some was also grown in Gloucestershire and other "Westerlie Parts" according to one source. The evidence for this comes from several angles including titherecords, estate records and field names. In Norfolk, customs records show locally grown saffron was exported to the Low Countries .[67] (The crop has recently been re-introduced to Norfolk and award-winning ISO 3632 category I saffron is grown at Burnham Norton. However, an influx of more exotic spices—chocolate, coffee, tea, and vanilla—from newly contacted Eastern and overseas countries caused European cultivation and usage of saffron to decline.[68][69] The last grower in England appears to have been John Knott of Duxford in Cambridgeshire, who delivered his crop to London apothecaries until around 1818 .[70] It would be nearly two centuries before saffron was commercially grown in England again. Only in southern France, Italy, and Spain did the clone significantly endure.[71] Europeans introduced saffron to the Americas when immigrant members of the Schwenkfelder Church left Europe with a trunk containing its corms. Church members had grown it widely in Europe.[37] By 1730, the Pennsylvania Dutch cultivated saffron throughout eastern Pennsylvania. Spanish colonies in the Caribbean bought large amounts of this new American saffron, and high demand ensured that saffron's list price on the Philadelphia commodities exchange was equal to gold.[72] Trade with the Caribbean later collapsed in the aftermath of the War of 1812, when many saffron-bearing merchant vessels were destroyed.[73] Yet the Pennsylvania Dutch continued to grow lesser amounts of saffron for local trade and use in their cakes, noodles, and chicken or trout dishes.[74] American saffron cultivation survives into modern times, mainly in Lancaster County, Pennsylvania.[37]
Trade and use
Main article: Trade and use of saffron
| NUTRITIONAL VALUE PER 100 G (3.5 OZ) | |
|---|---|
| ENERGY | 1,298 kJ (310 kcal) |
65.37 g
| |
| DIETARY FIBRE | 3.9 g |
5.85 g
| |
| SATURATED | 1.586 g |
| MONOUNSATURATED | 0.429 g |
| POLYUNSATURATED | 2.067 g |
11.43 g
| |
| VITAMINS | |
| VITAMIN A | 530 IU |
| THIAMINE (B1) |
(10%)
0.115 mg |
| RIBOFLAVIN (B2) |
(22%)
0.267 mg |
| NIACIN (B3) |
(10%)
1.460 mg |
| VITAMIN C |
(97%)
80.8 mg |
| TRACE METALS | |
| CALCIUM |
(11%)
111 mg |
| IRON |
(85%)
11.10 mg |
| MAGNESIUM |
(74%)
264 mg |
| PHOSPHORUS |
(36%)
252 mg |
| POTASSIUM |
(37%)
1724 mg |
| SODIUM |
(10%)
148 mg |
| ZINC |
(11%)
1.09 mg |
| OTHER CONSTITUENTS | |
| WATER | 11.90 g |
| SELENIUM | 5.6 μg |
| FOLATE[N 1] | 93 μg |
| VITAMIN B6 | 1.010 mg |
| ASH | 5.45 g |
Edible thread portion only.[75]
| |
| |
| Percentages are roughly approximated usingUS recommendations for adults. Source: USDA Nutrient Database | |
Trade
Almost all saffron grows in a belt from Spain in the west to India in the east. The other continents, except Antarctica, produce smaller amounts. Some 300 t (300,000 kg) of dried whole threads and powder are gleaned yearly,[14] of which 50 t (50,000 kg) is top-grade "coupe" saffron.[76] Iran answers for around 90–93% of global production and exports much of it.[16] A few of Iran's drier eastern and southeastern provinces, including Fars, Kerman, and those in the Khorasan region, glean the bulk of modern global production. In 2005, the second-ranked Greece produced 5.7 t (5,700 kg), while Morocco and Kashmir, tied for third rank, each produced 2.3 t (2,300 kg).[16] In recent years, Afghan cultivation has risen. Azerbaijan, Morocco, and Italy are, in decreasing order, lesser producers. Prohibitively high labour costs and abundant Iranian imports mean that only select locales continue the tedious harvest in Austria, Germany, and Switzerland—among them the Swiss village of Mund, whose annual output is a few kilograms.[14] Microscale production of saffron can be found in Tasmania,[77] China, Egypt, England (the village of Burnham Norton[78]) France, Israel, Mexico, New Zealand, Turkey (mainly around the town of Safranbolu), California, and Central Africa.[4][28] To glean 1 lb (450 g) of dry saffron requires the harvest of 50,000–75,000 flowers; a kilogram requires 110,000–170,000 flowers.[79][80] Forty hours of labour are needed to pick 150,000 flowers.[81] Stigmas are dried quickly upon extraction and (preferably) sealed in airtight containers.[82] Saffron prices at wholesale and retail rates range from US$500 to US$5,000 per pound, or US$1,100–11,000/kg, equivalent to £2,500/€3,500 per pound or £5,500/€7,500 per kilogram. In Western countries, the average retail price in 1974 was $1,000/£500/€700 per pound, or US$2,200/£1,100/€1,550 per kilogram.[4] In February 2013, a retail bottle containing 0.06 ounces could be purchased for $16.26 or the equivalent of $4,336 per pound or as little as about $2,000/pound in larger quantities. A pound contains between 70,000 and 200,000 threads. Vivid crimson colouring, slight moistness, elasticity, and lack of broken-off thread debris are all traits of fresh saffron.
Use
Saffron's aroma is often described by connoisseurs as reminiscent of metallic honey with grassy or hay-like notes, while its taste has also been noted as hay-like and sweet. Saffron also contributes a luminous yellow-orange colouring to foods. Saffron is widely used in Indian, Persian, European, Arab, and Turkish cuisines. Confectioneries and liquors also often include saffron. Common saffron substitutes include safflower (Carthamus tinctorius, which is often sold as "Portuguese saffron" or "açafrão"), annatto, and turmeric (Curcuma longa). Saffron has also been used as a fabric dye, particularly in China and India, and in perfumery.[83] It is used for religious purposes in India, and is widely used in cooking in many cuisines, ranging from the Milanese risotto of Italy to the bouillabaisse of France to the biryani with various meat accompaniments in South Asia. Saffron also has a long history of use in traditional medicine.[84]
Biomedical research
There is some evidence to suggest that saffron may help alleviate the symptoms of major depressive disorder.[85][86] Preclinical studies indicate that saffron could be a promising candidate for cancer chemoprevention studies.[87] Early studies suggest that it may protect the eye from the direct effects of bright light, and from retinal stress in additional to slowing down macular degeneration and retinitis pigmentosa.[88] (Most saffron-related research refers to the stigmas, but this is often not made explicit in research papers.) Some studies suggest that saffron may help relieve the symptoms of premenstrual syndrome.[89][90]
Notes
- "Folate" refers only to the naturally occurring form of folic acid; the sample contains no folic acid per se.[75]
Citations
- "Saffron – Definition and More". Merriam-Webster. Retrieved 21 November 2012.
- Kafi et al. 2006, p. 23.
- Rau 1969, p. 53.
- Hill 2004, p. 272.
- "World's COSTLIEST spice blooms in Kashmir".Rediff. Retrieved 7 January 2013.
- Grigg 1974, p. 287.
- McGee 2004, p. 422.
- Rubio-Moraga et al. 2009.
- ^ Jump up to:a b Negbi 1999, p. 28.
- ^ Jump up to:a b c d Caiola 2003, p. 1.
- Jump up^ Negbi 1999, p. 30–31.
- ^ Jump up to:a b Negbi 1999, p. 1.
- ^ Jump up to:a b McGee 2004, p. 423.
- ^ Jump up to:a b c d Katzer 2010.
- ^ Jump up to:a b Russo, Dreher & Mathre 2003, p. 6.
- ^ Jump up to:a b c Ghorbani 2008, p. 1.
- Deo 2003, p. 1.
- Jump up^ Kafi et al. 2006, p. 24.
- Willard 2002, p. 3.
- Jump up^ Government of Tasmania 2005.
- ^ Jump up to:a b Willard 2002, pp. 2–3.
- Jump up^ Deo 2003, p. 2.
- Jump up^ Sharaf-Eldin et al. 2008.
- ^ Jump up to:a b Deo 2003, p. 3.
- Jump up^ Willard 2002, pp. 3–4.
- Jump up^ Willard 2002, p. 4.
- ^ Jump up to:a b Deo 2003, p. 4.
- ^ Jump up to:a b c d Abdullaev 2002, p. 1.
- ^ Jump up to:a b Leffingwell 2002, p. 1.
- Jump up^ Dharmananda 2005.
- ^ Jump up to:a b Leffingwell 2002, p. 3.
- Jump up^ Verma & Middha 2010, p. 1–2.
- Jump up^ Hill 2004, p. 274.
- Jump up^ Willard 2002, pp. 102–104.
- Jump up^ Australian Broadcasting Corp. 2003.
- Jump up^ Hussain 2005.
- ^ Jump up to:a b c Willard 2002, p. 143.
- Jump up^ Willard 2002, p. 201.
- ^ Jump up to:a b Honan 2004.
- ^ Jump up to:a b c Willard 2002, p. 2.
- Jump up^ Humphries 1998, p. 20.
- Jump up^ Willard 2002, p. 12.
- Jump up^ Willard 2002, pp. 17–18.
- ^ Jump up to:a b Willard 2002, p. 41.
- Jump up^ Willard 2002, pp. 54–55.
- Jump up^ Lak 1998b.
- Jump up^ Fotedar 1999, p. 128.
- ^ Jump up to:a b Dalby 2002, p. 95.
- Jump up^ Dalby 2003, p. 256.
- Jump up^ Finlay 2003, p. 224.
- Jump up^ Hanelt 2001, p. 1352.
- Jump up^ Fletcher 2005, p. 11.
- Jump up^ Hayes 2001, p. 6.
- Jump up^ Ferrence & Bendersky 2004, p. 1.
- Jump up^ Willard 2002, p. 58.
- Jump up^ Willard 2002, p. 55.
- Jump up^ Willard 2002, pp. 34–35.
- Jump up^ Willard 2002, p. 59.
- Jump up^ Marx 1989.
- Jump up^ Willard 2002, p. 63.
- Jump up^ Willard 2002, p. 70.
- Jump up^ Willard 2002, p. 99.
- Jump up^ Willard 2002, p. 101.
- Jump up^ Willard 2002, pp. 103–104.
- Jump up^ Francis 2011, p. 17.
- Jump up^ Francis 2011, p. 21.
- Jump up^ Francis 2011, p. 33.
- Jump up^ Willard 2002, p. 117.
- Jump up^ Willard 2002, pp. 132–133.
- Jump up^ Francis 2011, p. 36.
- Jump up^ Willard 2002, p. 133.
- Jump up^ Willard 2002, p. 138.
- Jump up^ Willard 2002, pp. 138–139.
- Jump up^ Willard 2002, pp. 142–146.
- ^ Jump up to:a b United States Department of Agriculture.
- Jump up^ Negbi 1999, p. 2.
- Jump up^ Courtney 2002.
- Jump up^ "Norfolk Saffron; England’s ‘red gold’". Our Norfolk. Retrieved 22 January 2015.
- Jump up^ Hill 2004, p. 273.
- Rau 1969, p. 35.
- Jump up^ Lak 1998a.
- Negbi 1999, p. 8.
- Jump up^ Dalby 2002, p. 138.
- Mousavi, S. Z.; Bathaie, S. Z. (2011). "Historical uses of saffron: Identifying potential new avenues for modern research". Avicenna Journal of Phytomedicine 1 (2): 27–66.
- Jump up^ Hausenblas HA, Saha D, Dubyak PJ, Anton SD (November 2013). "Saffron (Crocus sativus L.) and major depressive disorder: a meta-analysis of randomized clinical trials". Journal of Integrative Medicine 11 (6): 377–83. doi:10.3736/jintegrmed2013056.PMID 24299602.
- Lopresti AL, Drummond PD (2014). "Saffron (Crocus sativus) for depression: a systematic review of clinical studies and examination of underlying antidepressant mechanisms of action". Human Psychopharmacology: Clinical and Experimental. doi:10.1002/hup.2434.
- Zhang Z, Wang CZ, Wen XD, Shoyama Y, Yuan CS (July 2013). "Role of saffron and its constituents on cancer chemoprevention". Pharmaceutical Biology 51(7): 920–4. doi:10.3109/13880209.2013.771190.PMC 3971062. PMID 23570520.
- Maccarone, Di Marco & Bisti 2008.
- Moghaddasi 2010.
- Dante G, Facchinetti F (March 2011). "Herbal treatments for alleviating premenstrual symptoms: a systematic review". Journal of Psychosomatic Obstetrics and Gynaecology 32 (1): 42–51.doi:10.3109/0167482X.2010.538102.PMID 21171936.
References
Books
- Bailes, M. (1995), The Healing Garden, Kangaroo Press, ISBN 978-0-86417-636-3
- Dalby, A. (2002), Dangerous Tastes: The Story of Spices (1st ed.), University of California Press, ISBN 978-0-520-23674-5
- Dalby, A. (2003), Food in the Ancient World from A to Z, Routledge, ISBN 978-0-415-23259-3
- Finlay, V. (2003), Colour: A Natural History of the Palette, Random House, ISBN 978-0-8129-7142-2
- Fletcher, N. (2005), Charlemagne's Tablecloth: A Piquant History of Feasting (1st ed.), Saint Martin's Press, ISBN 978-0-312-34068-1
- Francis, S. (2011), Saffron: The Story of England's Red Gold, With Delicious Saffron Recipes that Family and Friends will Love, Norfolk Saffron, ISBN 978-0-955-04667-4
- Grigg, D. B. (1974), The Agricultural Systems of the World (1st ed.), Cambridge University Press, ISBN 978-0-521-09843-4
- Hayes, A. W. (2001), Principles and Methods of Toxicology (4th ed.), Taylor and Francis,ISBN 978-1-56032-814-8
- Hill, T. (2004), The Contemporary Encyclopedia of Herbs and Spices: Seasonings for the Global Kitchen (1st ed.), Wiley, ISBN 978-0-471-21423-6
- Humphries, J. (1998), The Essential Saffron Companion, Ten Speed Press, ISBN 978-1-58008-024-8
- Kafi, M.; Koocheki, A.; Rashed, M. H.; Nassiri, M. (eds.) (2006), Saffron (Crocus sativus) Production and Processing (1st ed.), Science Publishers, ISBN 978-1-57808-427-2
- Kumar, V. (2006), The Secret Benefits of Spices and Condiments, Sterling Publishers,ISBN 978-1-84557-585-4
- Hanelt, P. (ed.) (2001), Mansfeld's Encyclopedia of Agricultural and Horticultural Crops (1st ed.), Springer, ISBN 978-3-540-41017-1
- Marx, F. (translator) (1989), Celsus: De Medicina, Loeb Classical Library L292 (1–4),Harvard University Press, ISBN 978-0-674-99322-8, retrieved 15 September 2011
- McGee, H. (2004), On Food and Cooking: The Science and Lore of the Kitchen, Scribner,ISBN 978-0-684-80001-1
- Negbi, M. (ed.) (1999), Saffron: Crocus sativus L., CRC Press, ISBN 978-90-5702-394-1
- Rau, S. R. (1969), The Cooking of India, Foods of the World, Time-Life Books, ISBN 978-0-8094-0069-0
- Russo, E.; Dreher, M. C.; Mathre, M. L. (2003), Women and Cannabis: Medicine, Science, and Sociology (1st ed.), Psychology Press, ISBN 978-0-7890-2101-4
- Willard, P. (2002), Secrets of Saffron: The Vagabond Life of the World's Most Seductive Spice, Beacon Press, ISBN 978-0-8070-5009-5
Journal articles
- Abdullaev, F. I. (2002), "Cancer Chemopreventive and Tumoricidal Properties of Saffron (Crocus sativus L.)", Experimental Biology and Medicine 227 (1), PMID 11788779, retrieved 11 September 2011
- Agha-Hosseini, M.; Kashani, L.; Aleyaseen, A.; Ghoreishi, A.; Rahmanpour, H.; Zarrinara, A. R.; Akhondzadeh, S. (2008), "Crocus sativus L. (Saffron) in the Treatment of Premenstrual Syndrome: A Double-Blind, Randomised, and Placebo-Controlled Trial", BJOG: An International Journal of Obstetrics and Gynaecology 115 (4): 515–519, doi:10.1111/j.1471-0528.2007.01652.x, PMID 18271889
- Akhondzadeh, S.; Sabet, M. S.; Harirchian, M. H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S. S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; Zare, F.; Moradi, A. (2010), "Saffron in the Treatment of Patients with Mild to Moderate Alzheimer's Disease: A 16-week, Randomised, and Placebo-Controlled Trial", Journal of Clinical Pharmacy and Therapeutics 35(5): 581–588, doi:10.1111/j.1365-2710.2009.01133.x, PMID 20831681
- Assimopoulou, A. N.; Papageorgiou, V. P.; Sinakos, Z. (2005), "Radical Scavenging Activity ofCrocus sativus L. Extract and Its Bioactive Constituents", Phytotherapy Research 19 (11),doi:10.1002/ptr.1749, PMID 16317646
- Boskabady, M. H.; Ghasemzadeh Rahbardar, M.; Nemati, H.; Esmaeilzadeh, M. (2010), "Inhibitory Effect of Crocus sativus (Saffron) on Histamine (H1) Receptors of Guinea Pig Tracheal Chains", Die Pharmazie 65 (4): 300–305, PMID 20432629
- Caiola, M. G. (2003), "Saffron Reproductive Biology", Acta Horticulturae (ISHS) 650: 25–37
- Chang, P. Y.; Kuo, W.; Liang, C. T.; Wang, C. K. (1964), "The Pharmacological Action of 藏红花 (Zà Hóng Huā—Crocus sativus L.): Effect on the Uterus and Estrous Cycle", Yao Hsueh Hsueh Pao 11
- Chryssanthi, D. G.; Dedes, P. G.; Karamanos, N. K.; Cordopatis, P.; Lamari, F. N. (2011), "Crocetin Inhibits Invasiveness of MDA-MB-231 Breast Cancer Cells via Downregulation of Matrix Metalloproteinases", Planta Medica 77 (2): 146–151, doi:10.1055/s-0030-1250178,PMID 20803418
- Das, I.; Das, S.; Saha, T. (2010), "Saffron Suppresses Oxidative Stress in DMBA-Induced Skin Carcinoma: A Histopathological Study", Acta Histochemica 112 (4): 317–327,doi:10.1016/j.acthis.2009.02.003, PMID 19328523
- Davies, N. W.; Gregory, M. J.; Menary, R. C. (2005), "Effect of Drying Temperature and Air Flow on the Production and Retention of Secondary Metabolites in Saffron", Journal of Agricultural and Food Chemistry 53 (15): 5969–5975, doi:10.1021/jf047989j,PMID 16028982
- Deo, B. (2003), "Growing Saffron—The World's Most Expensive Spice", Crop and Food Research (New Zealand Institute for Crop and Food Research) (20), archived from the original on 27 December 2005, retrieved 10 January 2006
- Dharmananda, S. (2005), "Saffron: An Anti-Depressant Herb", Institute for Traditional Medicine, archived from the original on 26 September 2006, retrieved 10 January 2006
- Ferrence, S. C.; Bendersky, G. (2004), "Therapy with Saffron and the Goddess at Thera",Perspectives in Biology and Medicine 47 (2): 199–226, doi:10.1353/pbm.2004.0026,PMID 15259204
- Ghorbani, M. (2008), "The Efficiency of Saffron's Marketing Channel in Iran", World Applied Sciences Journal 4 (4): 523–527, ISSN 1818-4952, retrieved 3 October 2011
- Gout, B.; Bourges, C.; Paineau-Dubreuil, S. (2010), "Satiereal, a Crocus sativus L. Extract, Reduces Snacking and Increases Satiety in a Randomised Placebo-Controlled Study of Mildly Overweight, Healthy Women", Nutrition Research 30 (5): 305–313,doi:10.1016/j.nutres.2010.04.008, PMID 20579522
- Gutheil, W. G.; Reed, G.; Ray, A.; Dhar, A. (2011), "Crocetin: An Agent Derived from Saffron for Prevention and Therapy for Cancer", Current Pharmaceutical Biotechnology,PMID 21466430
- Hasegawa, J. H.; Kurumboor, S. K.; Nair, S. C. (1995), "Saffron Chemoprevention in Biology and Medicine: A Review", Cancer Biotherapy 10 (4), PMID 8590890
- Hausenblas, H. A.; Saha, D.; Dubyakt, P. A.; Anton, P. J. (2013), "Saffron (Crocus sativus L.) and major depressive disorder: a meta-analysis of randomized clinical trials", Journal of Integrative Medicine 11 (6), doi:10.3736/jintegrmed2013056, PMID 24299602
- Hosseinzadeh, H.; Karimi, G.; Niapoor, M. (2004), "Antidepressant Effect of Crocus sativus L. Stigma Extracts and Their Constituents, Crocin and Safranal, In Mice", Acta Horticulturae(International Society for Horticultural Science) (650): 435–445, retrieved 23 November 2009
- Jessie, S. W.; Krishnakantha, T. P. (2005), "Inhibition of Human Platelet Aggregation and Membrane Lipid Peroxidation by Saffron", Molecular and Cellular Biochemistry 278 (1–2): 59–63, doi:10.1007/s11010-005-5155-9, PMID 16180089
- Joukar, S.; Najafipour, H.; Khaksari, M.; Sepehri, G.; Shahrokhi, N.; Dabiri, S.; Gholamhoseinian, A.; Hasanzadeh, S. (2010), "The Effect of Saffron Consumption on Biochemical and Histopathological Heart Indices of Rats with Myocardial Infarction",Cardiovascular Toxicology 10 (1): 66–71, doi:10.1007/s12012-010-9063-1,PMID 20119744
- Kianbakht, S.; Ghazavi, A. (2011), "Immunomodulatory Effects of Saffron: A Randomized Double-Blind Placebo-Controlled Clinical Trial", Phytotherapy Research,doi:10.1002/ptr.3484, PMID 21480412
- Lopresti, A. L.; Drummond, P. D. (2014), "Saffron (Crocus sativus) for depression: a systematic review of clinical studies and examination of underlying antidepressant mechanisms of action",Human Psychopharmacology: Clinical and Experimental, doi:10.1002/hup.2434
- Moghaddasi, M. S. (2010), "Saffron Chemicals and Medicine Usage" (PDF), Journal of Medicinal Plant Research 4 (6): 427–430, retrieved 30 September 2011
- Maccarone, R.; Di Marco, S.; Bisti, S. (2008), "Saffron Supplement Maintains Morphology and Function after Exposure to Damaging Light in Mammalian Retina", Investigative Ophthalmology and Visual Science 49 (3): 1254–1261, doi:10.1167/iovs.07-0438,PMID 18326756
- Nair, S. C.; Pannikar, B.; Panikkar, K. R. (1991), "Antitumour Activity of Saffron (Crocus sativus).", Cancer Letters 57 (2), doi:10.1016/0304-3835(91)90203-T, PMID 2025883
- Rubio-Moraga, A.; Castillo-López, R.; Gómez-Gómez, L.; Ahrazem, O. (2009), "Saffron is a Monomorphic Species as Revealed by RAPD, ISSR and Microsatellite Analyses", BMC Research Notes 2: 189, doi:10.1186/1756-0500-2-189, PMC 2758891, PMID 19772674
- Sharaf-Eldin, M.; Elkholy, S.; Fernández, J. A.; Junge, H.; Cheetham, R.; Guardiola, J.; Weathers, P. (2008), "Bacillus subtilis FZB24 Affects Flower Quantity and Quality of Saffron (Crocus sativus)", Planta Med 74 (10): 1316–1320, doi:10.1055/s-2008-1081293,PMC 3947403, PMID 18622904
- Verma, R. S.; Middha, D. (2010), "Analysis of Saffron (Crocus sativus L. Stigma) Components by LC–MS–MS", Chromatographia 71 (1–2): 117–123, doi:10.1365/s10337-009-1398-z
Miscellaneous
- Courtney, P. (2002), "Tasmania's Saffron Gold", Landline (Australian Broadcasting Corp., published 19 May 2002), retrieved 29 September 2011
- Fotedar, S. (1999), "Cultural Heritage of India: The Kashmiri Pandit Contribution", Vitasta(Kashmir Sabha of Kolkata) 32 (1), retrieved 15 September 2011
- Harper, D. (2001), Online Etymology Dictionary, retrieved 12 September 2011
- Honan, W. H. (2004), "Researchers Rewrite First Chapter for the History of Medicine", The New York Times (2 March 2004), retrieved 13 September 2011
- Hussain, A. (2005), Saffron Industry in Deep Distress, London: BBC News (published 28 January 2005), retrieved 15 September 2011
- Katzer, G. (2010), "Saffron (Crocus sativus L.)", Gernot Katzer's Spice Pages, retrieved1 December 2012
- Lak, D. (1998), "Kashmiris Pin Hopes on Saffron", BBC News (11 November 1998), retrieved11 September 2011
- Lak, D. (1998), "Gathering Kashmir's Saffron", BBC News (23 November 1998), retrieved12 September 2011
- Leffingwell, J. C. (2002), "Saffron" (PDF), Leffingwell Reports (October 2002) 2 (5), retrieved 15 September 2011
- Malik, N. (2007), Saffron Manual for Afghanistan, DACAAR Rural Development Program, retrieved 17 September 2011
- Park, J. B. (2005), "Saffron", USDA Phytochemical Database, archived from the originalon 25 September 2006, retrieved 10 January 2006
Other
- Kashmiri Saffron Producers See Red over Iranian Imports, Australian Broadcasting Corp.(published 4 November 2003), 2003, retrieved 29 September 2011
- "Emerging and Other Fruit and Floriculture: Saffron", Food and Agriculture (Department of Primary Industries, Water, and Environment (DPIWE), Government of Tasmania), 2005
- "Saffron", USDA National Nutrient Database (United States Department of Agriculture), retrieved 30 September 2011
External links
- "Saffron", Darling Biomedical Library (UCLA)
- "Crocus sativus", Germplasm Resources Information Network (USDA)
Contraindications, Interactions, and Side Effects (Saffron)
Saffron use in large dose is contraindicated in pregnancy. It may cause contraction of
Contraindications, Interactions, and Side Effects (Saffron)
Saffron use in large dose is contraindicated in pregnancy. It may cause contraction of uterus and abortion. Severe side effects may result from ingesting 5 g saffron. No side-effect when used in proper doses.
 flower
flower 











.gif)
 Chemical structure of ixazomib
Chemical structure of ixazomib








 http://infochem.de/
http://infochem.de/

 InfoChem will be represented at the forthcoming ACS Meeting in San Diego. You will find Dr. Josef Eiblmaier, Dr. Valentina Eigner Pitto, and Dr. Peter Loew …
InfoChem will be represented at the forthcoming ACS Meeting in San Diego. You will find Dr. Josef Eiblmaier, Dr. Valentina Eigner Pitto, and Dr. Peter Loew …









 Historische Bilder der Landsberger Straße – An der Trambahnhaltestelle Holzapfelstraße endet – Münchner Straßen – München – Süddeutsche.de
Historische Bilder der Landsberger Straße – An der Trambahnhaltestelle Holzapfelstraße endet – Münchner Straßen – München – Süddeutsche.de