Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, helping millions with websites, 9 million hits on google, 2.5 lakh connections worldwide, P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
Friday, 14 June 2013
Thursday, 13 June 2013
AstraZeneca buys Pearl Therapeutics in $1.15bn deal
AstraZeneca buys Pearl Therapeutics in $1.15bn deal
The UK's second largest drug company, AstraZeneca, has announced that it will buy US based lung disease drug specialist, Pearl Therapeutics, in a deal worth up to $1.15bn (£742m).
http://www.pharmaceutical-technology.com/news/newsastrazeneca-buys-pearl-therapeutics-in-115bn-deal?WT.mc_id=DN_News

PFIZER, MERCK, SANOFI --Does This Pipeline Promise Tomorrow's Blockbuster Sales?

Does This Pipeline Promise Tomorrow's Blockbuster Sales?
Motley Fool
Pfizer's future looks strong with its robust pipeline that boasts 74 total drugs in development, and at least in late-stage candidates, Merck looks strong as well, with another 23 therapies in phase 2 development. However, it's the quality, not the ...
read all at
http://www.fool.com/investing/general/2013/06/12/does-this-pipeline-promise-tomorrows-blockbuster-s.aspx


Wednesday, 12 June 2013
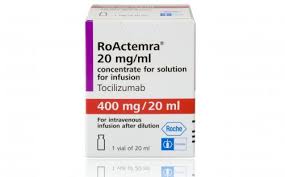
Roche's RoActemra gets EU OK for rare child arthritis

The European Medicines Agency has expanded approval for Roche's RoActemra to cover the treatment of children with polyarticular juvenile idiopathic arthritis.
The regulator has decreed that RoActemra (tocilizumab) can be used to treat patients two years of age and older who have not responded adequately to treatment with methotrexate. The drug can be given alone or in combination with MTX.

Tocilizumab(INN, or atlizumab, developed by Hoffmann–La Roche and Chugai and sold under the trade names Actemra and RoActemra) is an immunosuppressive drug, mainly for the treatment of rheumatoid arthritis (RA) and systemic juvenile idiopathic arthritis, a severe form of RA in children. It is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R). Interleukin 6 (IL-6) is a cytokine that plays an important role in immune response and is implicated in the pathogenesis of many diseases, such asautoimmune diseases, multiple myeloma and prostate cancer.



Will nanorods be the next big male contraceptive idea?

| |
Successful experiments on mice bode well for a future human contraceptive - if men can stomach the injections
Pet contraception is considered an important topic, given the four million unwanted dogs and cats that are thought to be put down every year in the US alone. Many vets routinely sterilise pets, but since surgery requires time and expertise scientists have been looking for cheaper, simpler alternatives. | |

Functionalising the nanorods with methoxy poly(ethylene glycol) enables them to be used for contraception or even sterilisation © ACS
New Fluzone Quadrivalent Four-Strain Influenza Vaccine from Sanofi Pasteur Now Licensed By FDA for Broad Age Range of Children and Adults
http://www.drugs.com/newdrugs/new-fluzone-quadrivalent-four-strain-influenza-vaccine-sanofi-pasteur-now-licensed-fda-broad-age-3810.html


Influenza (Flu)
90Y-Epratuzumab Study Shows Improvement of Therapy Results Following R-CHOP
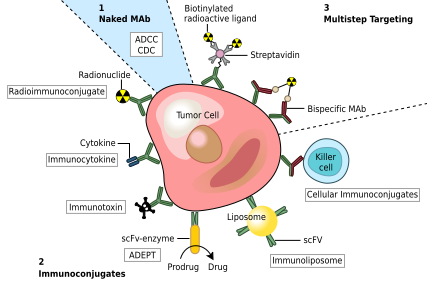
June 10, 2013 (GLOBE NEWSWIRE) -- Immunomedics, Inc. (Nasdaq:IMMU), a biopharmaceutical company primarily focused on the development of monoclonal antibody-based products for the targeted treatment of cancer, autoimmune and other serious diseases, today reported that adding two doses of epratuzumab labeled with the radioisotope, yttrium-90 (90Y), to a combination of rituximab and CHOP chemotherapy (R-CHOP), the standard of care for patients with diffuse large B-cell lymphoma (DLBCL), appeared to improve elderly patients' responses to treatment.
read all at
http://www.drugs.com/clinical_trials/90y-epratuzumab-study-shows-improvement-therapy-results-following-r-chop-15714.html
by
WORLD DRUG TRACKER
DR ANTHONY
Subscribe to:
Comments (Atom)