|
FEATURED
STORY
Peptide Has Promise for Treating Spinal Cord Injuryread athttp://www.dddmag.com/news/2014/12/peptide-has-promise-treating-spinal-cord-injury?et_cid=4300209&et_rid=523035093&type=cta |
|||||||||
|
Case
Western Reserve scientists have developed a new chemical compound that shows
extraordinary promise in restoring function lost to spinal cord injury. The
compound, which the researchers dubbed intracellular sigma peptide (ISP),
allowed paralyzed muscles to activate in more than 80 percent of the animals
tested. Read more...
|
Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, helping millions with websites, 9 million hits on google, 2.5 lakh connections worldwide, P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
Friday, 5 December 2014
Peptide Has Promise for Treating Spinal Cord Injury
Saturday, 29 November 2014
Antibacterial activities and antioxidant capacity of Aloe vera
Antibacterial activities and antioxidant capacity of Aloe vera
Fatemeh Nejatzadeh-Barandozi
- Correspondence: Fatemeh Nejatzadeh-Barandozifnejatzadeh@yahoo.com
Department of Horticulture, Faculty of Agriculture, Khoy Branch, Islamic Azad University, P.O. Box 58168–44799, Khoy, Ira
http://www.orgmedchemlett.com/content/3/1/5
http://www.orgmedchemlett.com/content/3/1/5
Organic and Medicinal Chemistry Letters 2013, 3:5 doi:10.1186/2191-2858-3-5
The electronic version of this article is the complete one and can be found online at:http://www.orgmedchemlett.com/content/3/1/5
Background
The aim of this study was to identify, quantify, and compare the phytochemical contents, antioxidant capacities, and antibacterial activities of Aloe vera lyophilized leaf gel (LGE) and 95% ethanol leaf gel extracts (ELGE) using GC-MS and spectrophotometric methods.
EU approves Lilly diabetes drug Trulicity, dulaglutide
EU approves Lilly diabetes drug Trulicity, dulaglutide
Regulators in Europe have given the green light to Eli Lilly’s Trulicity, its once-weekly glucagon-like peptide-1 receptor agonist for type 2 diabetes.
Read more at: http://www.pharmatimes.com/Article/14-11-25/EU_approves_Lilly_diabetes_drug_Trulicity.aspx
Dulaglutide is a glucagon-like peptide 1 receptor agonist (GLP-1 agonist) for the treatment of type 2 diabetes that can be used once weekly.[1][2]GLP-1 is a hormone that is involved in the normalization of level of glucose in blood (glycemia). The FDA approved dulaglutide for use in the United States in September 2014.[3] The drug is manufactured by Eli Lilly under the brand name Trulicity.[3]

Mechanism of action
Dulaglutide binding to glucagon-like peptide 1 receptor, slows gastric emptying and increases insulin secretion by beta cells in the pancreas. Simultaneously the compound reduces the elevated glucagon secretion by alpha cells of the pancreas, which is known to be inappropriate in the diabetic patient. GLP-1 is normally secreted by L cells of the gastrointestinal mucosa in response to a meal.[4]
Medical uses[
The compound is indicated for adults with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycemic control. Dulaglutide is not indicated in the treatment of subjects with type 1 diabetes mellitus or patients with diabetic ketoacidosis. Dulaglutide can be used either stand-alone or in combination with other medicines for type 2 diabetes, in particularmetformin, sulfonylureas, thiazolidinediones, and insulin taken concomitantly with meals.[5]
Side effects
The most common side effects include gastrointestinal disorders, such as dyspepsia,decreased appetite, nausea, vomiting, abdominal pain, diarrhea.[6] Some patients may experience serious adverse reactions: acute pancreatitis (symptoms include persistent severe abdominal pain, sometimes radiating to the back and accompanied by vomiting),hypoglycemia, renal impairment (which may sometimes require hemodialysis). The risk of hypoglycemia is increased if the drug is used in combination with sulfonylureasorinsulin.[7][8]
Contraindications
The compound is contraindicated in subjects with hypersensitivity to active principle or any of the product’s components. As a precautionary measure patients with a personal or family history of medullary thyroid carcinoma or affected by multiple endocrine neoplasia syndrometype 2 should not take dulaglutide, because for now it is unclear whether the compound can increase the risk of these cancers.[9]

References
- JCourtney Aavang Tibble, Tricia Santos Cavaiola, Robert R Henry (2013). “Longer Acting GLP-1 Receptor Agonists and the Potential for Improved Cardiovascular Outcomes: A Review of Current Literature”. Expert Rev Endocrinol Metab 8 (3): 247–259.doi:10.1586/eem.13.20.
- “Lilly’s Once-Weekly Dulaglutide Shows Non-Inferiority to Liraglutide in Head-to-Head Phase III Trial for Type 2 Diabetes”. Eli Lilly. Feb 25, 2014.
- “FDA approves Trulicity to treat type 2 diabetes” (Press release). FDA. Sep 18, 2014.
- Nadkarni P, Chepurny OG, Holz GG (2014). “Regulation of glucose homeostasis by GLP-1″. Prog Mol Biol Transl Sci 121: 23–65. doi:10.1016/B978-0-12-800101-1.00002-8.PMC 4159612. PMID 24373234. Retrieved 2014-09-29.
- Terauchi Y, Satoi Y, Takeuchi M, Imaoka T (July 2014). “Monotherapy with the once weekly GLP-1 receptor agonist dulaglutide for 12 weeks in Japanese patients with type 2 diabetes: dose-dependent effects on glycaemic control in a randomised, double-blind, placebo-controlled study”. Endocr. J. PMID 25029955. Retrieved 2014-09-29.
- Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z (August 2014). “Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5)”. Diabetes Care 37 (8): 2149–58.doi:10.2337/dc13-2761. PMID 24742660.
- Amblee A (April 2014). “Dulaglutide for the treatment of type 2 diabetes”. Drugs Today50 (4): 277–89. doi:10.1358/dot.2014.50.4.2132740. PMID 24918645.
- Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E (February 2014). “Glucagon-like peptide-1 receptor agonists and pancreatitis: a meta-analysis of randomized clinical trials”. Diabetes Res. Clin. Pract. 103 (2): 269–75.doi:10.1016/j.diabres.2014.01.010.PMID 24485345.
- Samson SL, Garber A (April 2013). “GLP-1R agonist therapy for diabetes: benefits and potential risks”. Curr Opin Endocrinol Diabetes Obes 20 (2): 87–97.doi:10.1097/MED.0b013e32835edb32. PMID 23403741. Retrieved 2014-09-30.
| IDENTIFIERS | |
|---|---|
| CAS NUMBER | 923950-08-7 |
| ATC CODE | None |
| CHEMICAL DATA | |
| FORMULA | C2646H4044N704O836S18 |
| MOL. MASS | 59669.81 g/mol |
Read all about Organic Spectroscopy on ORGANIC SPECTROSCOPY INTERNATIONAL 

KEBUZONE…….An antirheumatic agent.
KEBUZONE…….An antirheumatic agent.
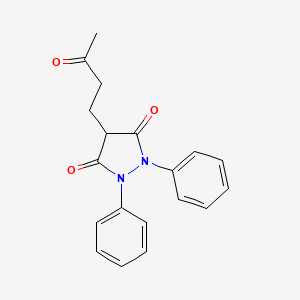
Kebuzone (or ketophenylbutazone) is a non-steroidal anti-inflammatory drug.
Structural formula
4-(3-oxobutyl)-1,2-diphenylpyrazolidine-3,5-dione
4-(3-Oxobutyl)-1,2-diphenyl-3,5-pyrazolidinedione
Additional Names: 1,2-diphenyl-4-(g-ketobutyl)-3,5-pyrazolidinedione; 1,2-diphenyl-4-(3¢-oxobutyl)-3,5-dioxopyrazolidine; ketophenylbutazone; KPB
Trademarks: Chebutan; Chepirol; Chetazolidin (Zeria); Chetil; Copirene; Ketason; Ketazone (Beytout); Pecnon (Sanken); Phloguron (Steiner); Recheton
MF: C19H18N2O3
MW: 322.36
Percent Comp: C 70.79%, H 5.63%, N 8.69%, O 14.89%
Properties: Crystals, mp 115.5-116.5° or 127.5-128.5° depending on cryst form.
Melting point: mp 115.5-116.5° or 127.5-128.5° depending on cryst form
Therap-Cat: Antirheumatic.
- BRN 0308507
- Chebutan
- Chepirol
- Chetazolidin
- Chetil
- Copirene
- EINECS 212-715-7
- Hichillos
- Kebuzone
- Kebuzonum
- Kebuzonum [INN-Latin]
- Keobutane-jade
- Ketason
- Ketazone
- Ketophenylbutazone
- Ketophenylbutazonum
- KPB
- Pecnon
- Quebuzona
- Quebuzona [INN-Spanish]
- Recheton
- UNII-4VD83UL6Y6
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions.They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects.
UV – range
IR – spectrum
Reference
- UV and IR Spectra. H.-W. Dibbern, R.M. Muller, E. Wirbitzki, 2002 ECV
- NIST/EPA/NIH Mass Spectral Library 2008
- Handbook of Organic Compounds. NIR, IR, Raman, and UV-Vis Spectra Featuring Polymers and Surfactants, Jr., Jerry Workman. Academic Press, 2000.
- Handbook of ultraviolet and visible absorption spectra of organic compounds, K. Hirayama. Plenum Press Data Division, 1967.
Brief background information
| SALT | ATC | FORMULA | MM | CAS |
|---|---|---|---|---|
| - | M01AA06 | C 19 H 18 N 2 O 3 | 322.36 g / mol | 853-34-9 |
| 4-(3-oxobutyl)-1,2-di(phenyl)pyrazolidine-3,5-dione | |
| CLINICAL DATA | |
|---|---|
| LEGAL STATUS |
?
|
| IDENTIFIERS | |
| CAS NUMBER | 853-34-9 |
| ATC CODE | M01AA06 |
| PUBCHEM | CID 3824 |
| CHEMSPIDER | 3692 |
| UNII | 4VD83UL6Y6 |
| KEGG | D01567 |
| CHEBI | CHEBI:31749 |
| CHEMICAL DATA | |
| FORMULA | C19H18N2O3 |
| MOL. MASS | 322.35782 g/mol |
Application
- anti-inflammatory
- antirheumatic
- Synthesis pathway
Trade names
| COUNTRY | TRADE NAME | MANUFACTURER |
|---|---|---|
| Germany | Kebuzon | Steiner |
| France | Ketazon | Beytout |
| Italy | Chetopir | Sarm |
| Ukraine | no | no |
Formulations
- ampoules of 1 g / 5 ml;
- 250 mg capsule
Reference
- Synthesis of a)
- Denss, R. et al .: Helv. Chim. Acta (HCACAV) 40, 402 (1957).
- material:
- Kühn, M .: J. Prakt. Chem. (JPCEAO) 156 (II), 103 (1940).
- Synthesis b)
- AT 198 263 (Synfarma; appl. 1955).
References: Prepn: Deuss et al., US 2910481 (1959 to Geigy).
Review of pharmacology: Horakova et al.,Pharmacotherapeutica 1950-1959, 335-350 (1963), C.A. 60, 6072g (1964).
Metabolism: Nemecek et al., Arzneim.-Forsch. 16,1339 (1966); Queisnerova, Nemecek,Cesk. Farm. 20, 55 (1971), C.A. 75, 47077u (1971).
Herrenknecht, Christine; Guernet-Nivaud, Elisabeth; Lafont, Olivier; Guernet, Michel; Gueutin, Claire
Canadian Journal of Chemistry, 1988 , v. 66, pg. 1199 – 1202
Canadian Journal of Chemistry, 1988 , v. 66, pg. 1199 – 1202
Cizmarik; Lycka
Pharmazie, 1988 , v. 43, 11 pg. 794 – 795
Pharmazie, 1988 , v. 43, 11 pg. 794 – 795
Gueutin-Pelinard, Claire; Nivaud, Elisabeth; Boucly, Patrick; Guernet, Michel
Canadian Journal of Chemistry, 1981 , v. 59, pg. 759 – 762
Canadian Journal of Chemistry, 1981 , v. 59, pg. 759 – 762
Denss et al.
Helvetica Chimica Acta, 1957 , v. 40, pg. 402,406
Helvetica Chimica Acta, 1957 , v. 40, pg. 402,406
Patent: CS124279 , 1965 ;Chem.Abstr., 1968 , v. 69, 52134r
SPOFA; United Pharmaceutical Work Patent: FR1500627 , 1965 ;Chem.Abstr., 1968 , v. 69, 96715k
Nippon Shinyaju Co., Ltd. Patent: US5811547 A1, 1998 ;
Fisnerova,L. et al. Collection of Czechoslovak Chemical Communications, 1974 , v. 39, pg. 624 – 633
Share this:
Subscribe to:
Comments (Atom)















