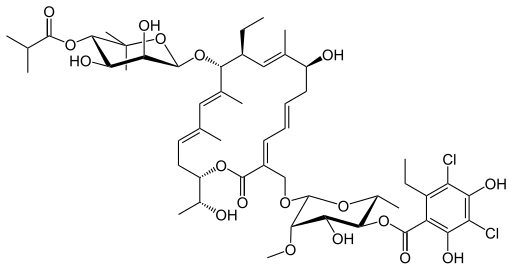
Fidaxomicin (C
52H
74Cl
2O
18, M
r = 1058.0 g/mol)
Launched - 2011 MERCK, Clostridium difficile-associated diarrhea
CUBIST ....INNOVATOR
OPT-80
PAR-101
Also tiacumicin B or lipiarmycin A3,
A bacterial RNA polymerase inhibitor as macrocyclic antibiotic used to treat clostridium difficile-associated diarrhea (CDAD).
SYNTHESIS
REFERENCES
US 4918174
WO 2006085838
J ANTIBIOTICS 1987, 40, PG 567-574 AND 575-588
Idaxomicin(trade names
Dificid,
Dificlir, and previously OPT-80 and PAR-101) is the first in a new class of narrow spectrum
macrocyclic antibiotic drugs.
[2] It is a fermentation product obtained from the actinomycete
Dactylosporangium aurantiacum subspecies hamdenesis.
[3][4] Fidaxomicin is non-systemic, meaning it is minimally absorbed into the bloodstream, it is
bactericidal, and it has demonstrated selective eradication of
pathogenic Clostridium difficile with minimal disruption to the multiple species of
bacteria that make up the normal, healthy
intestinal flora. The maintenance of normal physiological conditions in the colon can reduce the probability of
Clostridium difficile infection recurrence.
[5] [6]
Fidaxomicin
is an antibiotic approved and launched in 2011 in the U.S. for the
treatment of Clostridium difficile-associated diarrhea (CDAD) in adults
18 years of age and older. In September 2011, the product received a
positive opinion in the E.U. and final approval was assigned in December
2011.
First E.U. launch took place in the U.K. in June 2012.
Optimer Pharmaceuticals, now part of Cubist (now, Merck & Co.), is
conducting phase III clinical trials for the prevention of Clostridium
difficile-associated diarrhea in patients undergoing hematopoietic stem
cell transplant
In 2014 Astellas initiated in Europe a phase III
clinical study for the treatment of Clostridium difficile infection in
pediatric patients. Preclinical studies are ongoing for potential use in
the prevention of methicillin-resistant Staphylococcus (MRS) infection.


The
compound is a novel macrocyclic antibiotic that is produced by
fermentation. Its narrow-spectrum activity is highly selective for C.
difficile, thus preserving gut microbial ecology, an important
consideration for the treatment of CDAD.
It is marketed by Cubist
Pharmaceuticals after acquisition of its originating company Optimer
Pharmaceuticals. The target use is for treatment of
Clostridium difficile infection.
In
May 2005, Par Pharmaceutical and Optimer entered into a joint
development and collaboration agreement for fidaxomicin. However, rights
to the compound were returned to Optimer in 2007. The compound was
granted fast track status by the FDA in 2003. In 2010, orphan drug
designation was assigned to fidaxomicin in the U.S. by Optimer
Pharmaceuticals for the treatment of pediatric Clostridium difficile
infection (CDI). In 2011, the compound was licensed by Optimer
Pharmaceuticals to Astellas Pharma in Europe and certain countries in
the Middle East, Africa, the Commonwealth of Independent States (CIS)
and Japan for the treatment of CDAD. In 2011, fidaxomicin was licensed
to Cubist by Optimer Pharmaceuticals for comarketing in the U.S. for the
treatment of CDAD. In July 2012, the product was licensed by Optimer
Pharmaceuticals to Specialised Therapeutics Australia in AU and NZ for
the treatment of Clostridium difficile-associated infection. OBI Pharma
holds exclusive commercial rights in Taiwan, where the compound was
approved for the treatment of CDAD in September 2012, and in December
2012, the product was licensed to AstraZeneca in South America with
commercialization rights also for the treatment of CDAD. In October
2013, Optimer Pharmaceuticals was acquired by Cubist.
Fidaxomicin
is available in a 200 mg tablet that is administered every 12 hours for a
recommended duration of 10 days. Total duration of therapy should be
determined by the patient's clinical status. It is currently one of the
most expensive antibiotics approved for use. A standard course costs
upwards of £1350.
[7]
Fidaxomicin
(also known as OPT-80 and PAR-101 ) is a novel antibiotic agent and the
first representative of a new class of antibacterials called
macrocycles. Fidaxomicin is a member of the tiacumicin family, which are
complexes of 18-membered macrocyclic antibiotics naturally produced by a
strain of Dactylosporangium aurantiacum isolated from a soil sample
collected in Connecticut, USA.
The major component of the
tiacumicin complex is tiacumicin B. Optically pure R-tiacumicin B is the
most active component of Fidaxomicin. The chiral center at C(19) of
tiacumicinB affects biological activity, and R-tiacumicin B has an
R-hydroxyl group attached at this position. The isomer displayed
significantly higher activity than other tiacumicin B-related compounds
and longer post-antibiotic activity.
Tiacumicins are a family of structurally related compounds that contain the 18-membered macrolide ring shown below.
At present, several distinct Tiacumicins have been identified and six of these
(Tiacumicin A-F) are defined by their particular pattern of substituents R
1, R
2, and R
3 (US Patent No. 4,918,174; J. Antibiotics, 1987, 575-588).
The
Lipiarmycins are a family of natural products closely related to the
Tiacumicins. Two members of the Lipiarmycin family (A3 and B3) are
identical to Tiacumicins B and C respectively (J. Antibiotics, 1988,
308-315; J. Chem. Soc. Perkin Trans 1, 1987, 1353-1359).
The
Tiacumicins and the Lipiarmycins have been characterized by numerous
physical methods. The reported chemical structures of these compounds
are based on spectroscopy (UV-vis, IR and
!H and
13C NMR), mass spectrometry and elemental analysis (See for example: J. Antibiotics, 1987, 575-588; J. Antibiotics, 1983, 1312-
1322).
Tiacumicins
are produced by bacteria, including Dactylosporangium aurantiacum
subspecies hamdenensis, which may be obtained from the ARS Patent
Collection of the Northern Regional Research Center, United States
Department ofAgriculture, 1815 North University Street, Peoria, IL
61604, accession number NRRL
18085. The characteristics of strain AB 718C-41 are given in J. Antibiotics, 1987,567-574 and US Patent No. 4,918,174.
Lipiarmycins
are produced by bacteria including Actinoplanes deccanensis (US Patent
No. 3,978,211). Taxonomical studies of type strain A/10655, which has
been deposited in the ATCC under the number 21983, are discussed in J.
Antibiotics,1975, 247-25.
Tiacumicins, specifically Tiacumicin B,
show activity against a variety of bacterial pathogens and in particular
against Clostridium difficile, a Gram-positive bacterium (Antimicrob.
Agents Chemother. 1991, 1108-1111). Clostridium difficile is an
anaerobic spore-forming bacterium that causes an infection of the bowel.
As
per WIPO publication number 2006085838, Fidaxomicin is an isomeric
mixture of the configurationally distinct stereoisomers of tiacumicin B,
composed of 70 to 100% of R-tiacumicin B and small quantities of
related compounds, such as S-tiacumicin B and lipiarmycin A4.
Fidaxomicin was produced by fermentation of the D aurantiacum subspecies
hamdenensis (strain 718C-41 ). It has a narrow spectrum antibacterial
profile mainly directed against Clostridium difficile and exerts a
moderate activity against some other gram-positive species.
Fidaxomicin
is bactericidal and acts via inhibition of RNA synthesis by bacterial
RNA polymerase at a distinct site from that of rifamycins. The drug
product is poorly absorbed and exerts its activity in the
gastrointestinal (Gl) tract, which is an advantage when used in the
applied indication, treatment of C. difficile infection (CDI) (also
known as C. difficile-associated disease or diarrhoea [CDAD]).
Fidaxomicin is available as DIFICID oral tablet in US market.
Its
CAS chemical name is Oxacyclooctadeca-3,5,9, 13, 15-pentaen-2-one,
3-[[[6-deoxy-4-0-(3,5dichloro-2-ethyl-4,6-dihydroxybenzoyl)-2-0-methyl-P-D-manno
pyranosyl]oxy]methyl]-12[[6-deoxy-5-C-methyl-4-0-(2-methyl-1
-oxopropyl)- -D-lyxo-hexo pyranosyl]oxy]-1 1 -ethyl-8-hydroxy-18-[(1
R)-1 -hydroxyethyl] -9,13,15-trimethyl-, (3E.5E, 8S.9E.1 1 S.12R.13E,
15E.18S)-.
Structural formula (I) describes the absolute stereochemistry of fidaxomicin as determined by x-ray.

(I)
WIPO publication number 2004014295
discloses a process for preparation of Tiacumicins that comprises
fermentation of Dactylosporangium aurantiacum NRRL18085 in suitable
culture medium. It also provides process for isolation of tiacumicin
from fermentation broth using techniques selected from the group
consisting of: sieving and removing undesired material by eluting with
at least one solvent or a solvent mixture; extraction with at least one
solvent or a solvent mixture; Crystallization; chromatographic
separation; High-Performance Liquid Chromatography (HPLC); MPLC;
trituration; and extraction with saturated brine with at least one
solvent or a solvent mixture. The product was isolated from /so-propyl
alcohol (IPA) having a melting point of 166-169 °C.
U.S. Patent No. 7378508 B2
discloses polymorphic forms A and B of fidaxomicin, solid dosage forms
of the two forms and composition thereof. As per the ‘508 patent form A
is obtained from methanol water mixture and Form B is obtained from
ethyl acetate.
J. Antibiotics, vol. 40(5), 575-588 (1987)
discloses purification of Tiacumicins using suitable solvents wherein
tiacumicin B exhibited a melting point of 143-145 °C.
PCT application WO2013170142A1
describes three crystalline forms of Fidaxomicn namely, Form-Z, Form-Z1
and Form-C. IN2650/CHE/2013 describes 6 crystalline polymorphic forms
of Fidaxomicin namely, Forms I, Form la, Form II, Form Ha, Form III and
Form Ilia).
Mechanism
Fidaxomicin
binds to and prevents movement of the "switch regions" of bacterial
RNAP polymerase. Switch motion is important for opening and closing of
the DNA:RNA clamp, a process that occurs throughout RNA transcription
but especially during opening of double standed DNA during transcription
initiation.
[8] It has minimal systemic absorption and a narrow spectrum of activity; it is active against
Gram positive bacteria especially
clostridia. The minimal inhibitory concentration (MIC) range for
C. difficile (ATCC 700057) is 0.03–0.25 μg/mL.
[3]
Clinical trials
Good results were reported by the company in 2009 from a North American
phase III trial comparing it with oral
vancomycin for the treatment of
Clostridium difficile infection (CDI)
[9][10]
The study met its primary endpoint of clinical cure, showing that
fidaxomicin was non-inferior to oral vancomycin (92.1% vs. 89.8%). In
addition, the study met its secondary endpoint of recurrence: 13.3% of
the subjects had a recurrence with fidaxomicin vs. 24.0% with oral
vancomycin. The study also met its exploratory endpoint of global cure (77.7% for fidaxomicin vs. 67.1% for vancomycin).
[11] Clinical cure was defined as patients requiring no further CDI therapy two days after completion of study medication.
Global cure was defined as patients who were cured at the end of therapy and did not have a recurrence in the next four weeks.
[12]
Fidaxomicin
was shown to be as good as the current standard-of-care, vancomycin,
for treating CDI in a Phase III trial published in February 2011.
[13] The authors also reported significantly fewer recurrences of infection, a frequent problem with
C. difficile, and similar drug side effects.
Approvals and indications
For the treatment of
Clostridium difficile-associated diarrhea (CDAD), the drug won an FDA advisory panel's unanimous approval on April 5, 2011
[14] and full FDA approval on May 27, 2011.
[15]
PAPER
Enantioselective synthesis of putative lipiarmycin aglycon related to fidaxomicin/tiacumicin B
Angew Chem Int Ed 2015, 54(6): 1929
Enantioselective Synthesis of Putative Lipiarmycin Aglycon Related to Fidaxomicin/Tiacumicin B (pages 1929–1932)
Dr. William Erb, Dr. Jean-Marie Grassot, Dr. David Linder, Dr. Luc Neuville and Prof. Dr. Jieping Zhu
Article first published online: 24 NOV 2014 | DOI: 10.1002/anie.201409475

Chain gang:
In the synthesis of the title compound, the ene-diene ring-closing
metathesis was used for the formation of the 18-membered macrolactone
and the stereogenic centers of the molecule were installed by Brown's
alkoxyallylboration, allylation, and an Evans aldol reaction, while
iterative Horner–Wadsworth–Emmons reactions were used for chain
elongation.
http://onlinelibrary.wiley.com/doi/10.1002/anie.201409475/full
http://onlinelibrary.wiley.com/store/10.1002/anie.201409475/asset/supinfo/anie_201409475_sm_miscellaneous_information.pdf?v=1&s=75d40b6f8b214578d5a65518e7f384f03f377c35
PAPER
Total synthesis of the glycosylated macrolide antibiotic fidaxomicin
Org Lett 2015, 17(14): 3514
http://pubs.acs.org/doi/abs/10.1021/acs.orglett.5b01602
http://pubs.acs.org/doi/suppl/10.1021/acs.orglett.5b01602/suppl_file/ol5b01602_si_001.pdf
The
first enantioselective total synthesis of fidaxomicin, also known as
tiacumicin B or lipiarmycin A3, is reported. This novel glycosylated
macrolide antibiotic is used in the clinic for the treatment of Clostridium difficile
infections. Key features of the synthesis involve a rapid and
high-yielding access to the noviose, rhamnose, and orsellinic acid
precursors; the first example of a β-selective noviosylation; an
effective Suzuki coupling of highly functionalized substrates; and a
ring-closing metathesis reaction of a noviosylated dienoate precursor.
Careful selection of protecting groups allowed for a complete
deprotection yielding totally synthetic fidaxomicin.
The
identity of the synthetic compound to an authentic sample of
fidaxomicin (1) was confirmed by coinjection on RP-HPLC and an equimolar
mixed NMR-sample with an authentic sample. Rƒ = 0.44 (MeOH/CH2Cl2
1/10).
HRMS ESI calcd. for [C52H74Cl2NaO18] + [M+Na]+ : 1079.4144; found:1079.4151.
1H
NMR (600 MHz, Methanol-d4 , containing HCOO- ) δ 7.23 (d, J = 11.5 Hz,
1H), 6.60 (dd, J = 14.9, 11.8 Hz 1H), 5.95 (ddd, J = 14.7, 9.5, 4.8 Hz,
1H), 5.83 (s, 1H), 5.57 (ap t, J = 8.2 Hz, 1H), 5.14 (ap d, J = 10.7,
1H), 5.13 (dd, J = 9.7 Hz, 1H), 5.02 (d, J = 10.2 Hz, 1H), 4.74-4.70 (m,
1H), 4.71 (s, 1H), 4.64 (s, 1H), 4.61 (d, J = 11.6 Hz, 1H), 4.44 (d, J =
11.6 Hz, 1H), 4.22 (ap s, 1H), 4.02 (p, J = 6.3 Hz, 1H), 3.92 (dd, J =
3.2, 1.2 Hz, 1H), 3.75 (ddd, J = 13.9, 10.2, 3.3 Hz, 1H) 3.71 (d, J =
9.7 Hz 1H), 3.58-3.52 (m, 2H) 3.54 (s, 3H), 3.15-3.06 (m, 1H), 3.04-2.95
(m, 1H), 2.76-2.66 (m, 3H), 2.60 (hept, J= 7.0 Hz, 1H), 2.49 (ddd, J =
14.9, 9.5, 4.4 Hz, 1H), 2.43 (ddd, J = 13.8, 8.8, 4.5 Hz, 1H), 2.05-1.98
(m, 1H), 1.82 (d, J = 1.3 Hz, 3H), 1.76 (ap s, 3H), 1.66 (ap s, 3H),
1.32-1.27 (m, 4H), 1.22-1.15 (m, 12H), 1.15 (s, 3H), 1.13 (s, 3H), 0.88
(t, J = 7.4 Hz, 3H).
RP-HPLC tR =
14.87 min (A: H2O+0.1% HCOOH; Solvent B: MeCN+0.1% HCOOH; 1 mL/min; T =
20°C; B[%] (tR [min])= 10 (0 to 3); 100 (15).
PATENT
WO 2004014295
The term "Tiacumicin B" refers to molecule having the structure shown below:
Example 1
Dactylosporangium
aurantiacum subsp. hamdenensis AB 718C-41 NRRL 18085 (-20 °C stock),
was maintained on 1 mL of Medium No. 104 (Table 1). After standard
sterilization conditions (30 min., 121 °C, 1.05 kg/cm
2) the
seed flask (250 mL) containing Medium No. 104 (50 mL) was inoculated
with AB 718C-41 NRRL 18085 on a shaker (set @ 250 rpm) at 30 °C for 72
hr. Five percent vegetative inoculum from the first passage seed flask
was then transferred aseptically to a fermentation flask containing the
same ingredients as in Table 1.
Table 1: Ingredients of Medium No. 104

Fermentation
flasks were incubated on a rotary shaker at 30 °C for 3 to 12 days.
Samples of the whole culture fermentation broth were filtered. The
filter cake was washed with MeOH and solvents were removed under reduced
pressure. The residue was re-constituted in methanol to the same volume
of the original fermentation broth. Analysis was performed using a
Waters BREEZE HPLC system coupling with Waters 2487 2-channel UV/Vis
detector. Tiacumincins were assayed on a 50 x 4.6 μm I.D., 5 μm YMC
ODS-A column (YMC catalog # CCA AS05- 0546WT) with a mobile phase
consisting of 45% acetonitrile in water containing 0.1% phosphoric acid
at a flow rate of 1.5 mL/minute. Tiacumicins were detected at 266 nm. An
HPLC chromatogram of a crude product (Tiacumicin B retention time @
12.6 minutes) is shown in Fig. 1. In this example the crude yield of
Tiacumicin B was about 250 mg/L after 7 days. After purification by
HPLC, the yield of Tiacumicin B was about 100 mg/L.
Example 2
After standard sterilization conditions (30 min, 121 °C, 1.05 kg/cm
2)
the seed flask (250 mL) containing Medium No. 104 (50 mL) was
inoculated with AB 718C- 41 NRRL 18085 and incubated on a shaker (set @
250 rpm) at 30° C for 72 hr. Five percent vegetative inoculum from the
first passage seed flask was transferred aseptically to a seed flask
containing the same ingredients as in Table 1 and was incubated on a
rotary shaker at 30 °C for 72 hr. Five percent inoculum from the second
passage seed flasks was then used to inoculate with AB 718C-41 NRRL
18085 in a 5-liter fermenter containing Medium No. 104 (2.5 L).
Excessive foam formation was controlled by the addition of an
antifoaming agent (Sigma A-6426). This product is a mixture of
non-silicone organic defoamers in a polyol dispersion.
Glucose
consumption was monitored as a growth parameter and its level was
controlled by the addition of the feeding medium. Feeding medium and
conditions in Example 2 were as follows:
Feeding medium:
Fermenter Medium: No. 104
Fermenter Volume: 5 liters
Sterilization: 40 minutes, 121° C, 1.05 kg/cm
2
Incubation Temperature: 30 °C.
Aeration rate: 0.5-1.5 volumes of air per culture volume and minute
Fermenter Agitation: 300-500 rpm
The
fermentation was carried out for 8 days and the XAD-16 resin was
separated from the culture broth by sieving. After washing with water
the XAD-16 resin was eluted with methanol (5-10 x volume of XAD-16).
Methanol was evaporated and the oily residue was extracted three times
with ethyl acetate. The extracts were combined and concentrated under
reduced pressure to an oily residue. The oily residue was dried and
washed with hexane to give the crude product as a pale brown powder and
its HPLC chromatogram (Tiacumincin B rete tion time @ 11.8 minutes) is
shown in Figure 2. This was purified by silica gel column (mixture of
ethyl acetate and hexane as eluent) and the resultant material was
further purified by RP-HPLC (reverse phase HPLC) to give Tiacumicin B as
a white solid. The purity was determined to be >95% by HPLC
chromatography and the chromatogram (Tiacumincin B retention time @ 12.0
minutes) is shown in Figure 3. Analysis of the isolated Tiacumincin B
gave identical
!H and
13C NMR data to those
reported in J. Antibiotics, 1987, 575-588, and these are summarized
below. Tiacumicin B: mp 129-140 °C (white powder from RP-HPLC); mp
166-169 °C (white needles from isopropanol); [α]
D 20-6.9 (c 2.0, MeOH); MS m/z (ESI) 1079.7(M + Na)
+; H NMR (400 MHz, CD
3OD)
δ 7.21 (d, IH), 6.59 (dd, IH), 5.95 (ddd, IH), 5.83 (br s, IH), 5.57
(t, IH), 5.13 (br d, IH), 5.09 (t, IH), 5.02 (d, IH), 4.71 (m, IH), 4.71
(br s, IH), 4.64 (br s, IH), 4.61 (d, IH), 4.42 (d, IH), 4.23 (m, IH),
4.02 (pentet, IH), 3.92 (dd, IH), 3.73 (m, 2H), 3.70 (d, IH), 3.56 (s,
3H), 3.52-3.56 (m, 2H), 2.92 (m, 2H), 2.64-2.76 (m, 3H), 2.59 (heptet,
IH), 2.49 (ddd, IH), 2.42 (ddd, IH), 2.01 (dq, IH), 1.81 (s, 3H), 1.76
(s, 3H), 1.65 (s, 3H), 1.35 (d, 3H), 1.29 (m, IH), 1.20 (t, 3H), 1.19
(d, 3 H), 1.17 (d, 3H), 1.16 (d, 3H), 1.14 (s, 3H), 1.12 (s, 3H), 0.87
(t, 3H);
13C NMR (100 MHz, CD
3OD) δ 178.4, 169.7,
169.1, 154.6, 153.9, 146.2, 143.7, 141.9, 137.1, 137.0, 136.4, 134.6,
128.5, 126.9, 125.6, 124.6, 114.8, 112.8, 108.8, 102.3, 97.2, 94.3,
82.5, 78.6, 76.9, 75.9, 74.5, 73.5, 73.2, 72.8, 71.6, 70.5, 68.3, 63.9,
62.2, 42.5, 37.3, 35.4, 28.7, 28.3, 26.9, 26.4, 20.3, 19.6, 19.2, 18.7,
18.2, 17.6, 15.5, 14.6, 14.0, 11.4.
PATENT
http://www.google.com/patents/US7378508
macrolide of Formula I:

Structure of R-Tiacumicin B
The
structure of the R-Tiacumicin B (the major most active component) is
shown below in Formula I. The X-ray crystal structure of the
R-Tiacumicin B was obtained as a colorless, parallelepiped-shaped
crystal (0.08×0.14×0.22 mm) grown in aqueous methanol. This x-ray
structure confirms the structure shown below. The official chemical name
is
3-[[[6-Deoxy-4-O-(3,5-dichloro-2-ethyl-4,6-dihydroxybenzoyl)-2-O-methyl-β-D-mannopyranosyl]oxy]-methyl]-12(R)-[[6-deoxy-5-C-methyl-4-O-(2-methyl-1-oxopropyl)-β-D-lyxo-hexopyranosyl]oxy]-11(S)-ethyl-8(S)-hydroxy-18(S)-(1(R)-hydroxyethyl)-9,13,15-trimethyloxacyclooctadeca-3,5,9,13,15-pentaene-2-one.
7.2.1 Analytical Data of R-Tiacumicin B
The analytical data of R-Tiacumicin B (which is almost entirely (i.e., >90%) R-Tiacumicin).
mp 166-169° C. (white needle from isopropanol);
[α]
D 20-6.9 (c 2.0, MeOH);
MS m/z (ESI) 1079.7(M+Na)
+;
1H NMR (400 MHz, CD
3OD)
δ 7.21 (d, 1H), 6.59 (dd, 1H), 5.95 (ddd, 1H), 5.83 (br s, 1H), 5.57
(t, 1H), 5.13 (br d, 1H), 5.09 (t, 1H), 5.02 (d, 1H), 4.71 (m, 1H), 4.71
(br s, 1H), 4.64 (br s, 1H), 4.61 (d, 1H), 4.42 (d, 1H), 4.23 (m, 1H),
4.02 (pentet, 1H), 3.92 (dd, 1H), 3.73 (m, 2H), 3.70 (d, 1H), 3.56 (s,
3H), 3.52-3.56 (m, 2H), 2.92 (m, 2H), 2.64-2.76 (m, 3H), 2.59 (heptet,
1H), 2.49 (ddd, 1H), 2.42 (ddd, 1H), 2.01 (dq, 1H), 1.81 (s, 3H), 1.76
(s, 3H), 1.65 (s, 3H), 1.35 (d, 3H), 1.29 (m, 1H), 1.20 (t, 3H), 1.19
(d, 3H), 1.17 (d, 3H), 1.16 (d, 3 H), 1.14 (s, 3H), 1.12 (s, 3H), 0.87
(t, 3H);
13C NMR (100 MHz, CD
3OD) δ 178.4,
169.7, 169.1, 154.6, 153.9, 146.2, 143.7, 141.9, 137.1, 137.0, 136.4,
134.6, 128.5, 126.9, 125.6, 124.6, 114.8, 112.8, 108.8, 102.3, 97.2,
94.3, 82.5, 78.6, 76.9, 75.9, 74.5, 73.5, 73.2, 72.8, 71.6, 70.5, 68.3,
63.9, 62.2, 42.5, 37.3, 35.4, 28.7, 28.3, 26.9, 26.4, 20.3, 19.6, 19.2,
18.7, 18.2, 17.6, 15.5, 14.6, 14.0, 11.4.
PATENT
WO2013170142
EXAMPLES
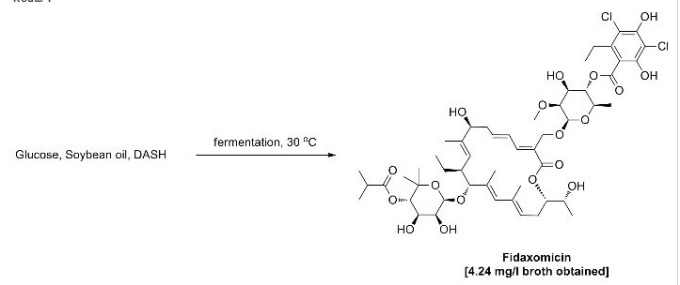
Example 1; General procedure for the preparation of crude Fidaxomycin
Fidaxomycin was prepared by:
i) culturing a microorganism in a nutrient medium to accumulate Fidaxomycin in the nutrient medium;
ii) isolating crude Fidaxomycin from the nutrient medium by methods known from the art;
iii)
purifying Fidaxomycin by reversed phase chromatography using a mixture
of acetonitrile, water and acetic acid as eluent; and iv) isolating the
purified Fidaxomycin from the fractions.
Actionplanes deccanenesis
was used during the cultivation. The nutrient medium comprises the
following combination based on weight: from about 0% to about 5%
Sucrose; from about 0% to about 3% Starch; from about 0.1% to about 1.0 %
Soy peptone; from about 2% to about 5% Cotton seed meal; from about
0.01% to about 0.1% Potassium-dihydrogen Phosphate; from about 0.05% to
about 0.5% Dipotassium-hydrogen Phosphate; from about 0.05% to about
0.5% Antifoam agent; from about 0% to about 2% Amberlite XAD-16N resin.
The preferred temperature of the cultivation is from 28 to 32°C, and the
pH is between 6.0 and 8.0. During the cultivation C-source is
continuously fed.
The Fidaxomycin fermentation production can also be done by the following procedure:
The Fidaxomycin fermentation production can include a step of inoculation followed by fermentation as follows:
Inoculation:
Actinoplanes deccanenesis strain is inoculated into the seed medium.
The inoculation parameters are adjusted and maintained until the
inoculum transferred to the main fermentation. The inoculum medium
comprises: from about 0 to about 5% glucose, from about 0 to about 1%
yeast extract, from about 0 to about 1% soy peptone, from about 0 to
about 0.5% CaCo
3, from about 0 to about 0.2% MgS0 -7H
20, from about 0 to about 0.2% K
2HP0
4,
from about 0 to about 0.2% KC1, from about 0 to about 0.3%
Polypropylene glycol. The pH is adjusted by adding Hydrochloric acid
and/or Sodium/potassium hydroxide.
Inoculation parameters :
Inoculation time: 40-48 ± 24 hours.
At
the end of the inoculation, the inoculum (or a part of it) is
transferred into the sterile fermentation medium at a ratio of 8-15 ± 5
%.
Fermentation: the fermentation medium comprises: from about 0
to aboutl0% Sucrose/Hydrolyzed Starch, from about 0 to about 1% Soy
peptone, from about 0 to about 5% Cotton seed meal, from about 0 to
about 0.3% K
2HP0
4, from about 0 to about 0.2% KH
2P0
4,
from about 0 to aboutl% KC1, from about 0 to about 0.5% Polypropylene
glycol (PPG). The pH is adjusted by adding Hydrochloric acid and/or
Sodium/potassium hydroxide.
The sterile fermentation medium is seeded with the inoculum.
Feeding:
C-source
is fed during the fermentation, For C-source feeding sucrose or
hydrolyzed-starch can be applied. Total amount of fed C-source is 0 -
15% related to the initial volume.
Fermentation parameters :
In case of foaming, sterile antifoaming agent should be added.
Fermentation time: 168-192 ± 24 hours.
The inoculation/fermentation medium may also include from about 0% to about 2% Amberlite XAD-16N resin.
Upon
completion of fermentation, the Fidaxomycin is extracted from the
fermented broth with an organic solvent such as, for example, ethyl
acetate, isobutyl acetate or isobutanol. The organic phase is
concentrated and the Fidaxomycin is precipitated by addition of an
antisolvent such as, for example, n-hexane. Optionally the precipitate
can be suspended in a second antisolvent. After filtration and drying,
crude Fidaxomycin is obtained.
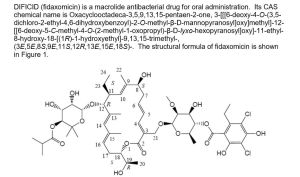
DIFICID
(fidaxomicin) is a macrolide antibacterial drug for oral
administration. Its CAS chemical name is
Oxacyclooctadeca-3,5,9,13,15-pentaen-2-one,
3-[[[6-deoxy-4-O-(3,5-dichloro-2-ethyl-4,6-dihydroxybenzoyl)-2-Omethyl-
β-D-
mannopyranosyl]oxy]methyl]-12-[[6-deoxy-5-C-methyl-4-O-(2-methyl-1-oxopropyl)-β-D-lyxohexopyranosyl]
oxy]-11-ethyl-8
-hydroxy-18-[(1R)-1-hydroxyethyl]-9,13,15-trimethyl-,(3E,5E,8S,9E,11S,12R,13E,15E,18S)-.
The structural formula of fidaxomicin is shown in Figure 1.
Figure 1: Structural Formula of Fidaxomicin





Patent
WO 2016024243, New patent, Dr Reddy’s Laboratories Ltd, Fidaxomicin
WO2016024243, FIDAXOMICIN POLYMORPHS AND PROCESSES FOR THEIR PREPARATION
DR. REDDY’S LABORATORIES LIMITED [IN/IN]; 8-2-337, Road No. 3, Banjara Hills, Telangana State, India Hyderabad 500034 (IN)
CHENNURU, Ramanaiah; (IN).
PEDDY, Vishweshwar; (IN).
RAMAKRISHNAN, Srividya; (IN)
Aspects
of the present application relate to crystalline forms of Fidaxomicin
IV, V & VI and processes for their preparation. Further aspects
relate to pharmaceutical compositions comprising these polymorphic forms
of fidaxomicin

The
occurrence of different crystal forms, i.e., polymorphism, is a
property of some compounds. A single molecule may give rise to a variety
of polymorphs having distinct crystal structures and physico-chemical
properties.
Polymorphs are different solid materials having the
same molecular structure but different molecular arrangement in the
crystal lattice, yet having distinct physico-chemical properties when
compared to other polymorphs of the same molecular structure. The
discovery of new polymorphs and solvates of a pharmaceutical active
compound provides an opportunity to improve the performance of a drug
product in terms of its bioavailability or release profile in vivo, or
it may have improved stability or advantageous handling properties.
Polymorphism is an unpredictable property of any given compound. This
subject has been reviewed in recent articles, including A. Goho, “Tricky
Business,” Science News, August 21 , 2004. In general, one cannot
predict whether there will be more than one form for a compound, how
many forms will eventually be discovered, or how to prepare any
previously unidentified form.
There remains a need for additional
polymorphic forms of fidaxomicin and for processes to prepare
polymorphic forms in an environmentally-friendly, cost-effective, and
industrially applicable manner.

G.V. Prasad, chairman, Dr Reddy’s Laboratories
EXAMPLES
Example 1 : Preparation of fidaxomicin Form IV:
Fidaxomicin
(0.5 g) and a mixture of 1 ,4-Dioxane (10 mL), THF (10 ml) and water
(20mL) were charged in Easy max reactor (Mettler Toledo). The reactor
was set to temperature cycle with following parameters:
Starting temperature: 25 °C;
Temperature raised to 60 °C over a period of 2 hours;
Cooled to 0 °C over a period of 2 hours;
Temperature raised to 60 °C over a period of 2 hours;
Cooled to 0 °C over a period of 2 hours;
Temperature raised to 25 °C over a period of 2 hours;
Temperature maintained at 25 °C for 6 hours.
After
completion of temperature cycling process, the slurry was filtered
under suction, followed by drying in air tray dryer (ATD) at 40
°C to a constant weight to produce crystalline fidaxomicin form-IV.
Example 2: Preparation of fidaxomicin Form V:
Fidaxomicin
(1 g) and a mixture of propylene glycol (10 mL) and water (20mL) were
charged in Easy max reactor (Mettler Toledo). The reactor was set to
temperature cycle with following parameters:
Starting temperature is 25 °C;
Temperature raised to 60 °C over a period of 2 hours;
Cooled to 0 °C over a period of 2 hours;
Temperature raised to 60 °C over a period of 2 hours;
Cooled to 0 °C over a period of 2 hours;
Temperature raised to 25 °C over a period of 2 hours;
Temperature maintained at 25 °C for 6 hours.
After
completion of temperature cycling process, the slurry was filtered
under suction, followed by drying in air tray dryer (ATD) at 40°C to a
constant weight to produce crystalline fidaxomicin form-V.
Example 3: Preparation of fidaxomicin Form VI:
Fidaxomicin
(0.5 mg) and MIBK (10 mL) were charged in Easy max reactor (Mettler
Toledo) and the mixture was heated to 80°C. n-heptane (20 mL) was added
to the solution at the same temperature. The mixture was stirred for 1
hour. The reaction mass was then cooled to 25°C. Solid formed was
filtered at 25°C and dried at 40°C in air tray dryer (ATD) to a constant
weight to produce crystalline fidaxomicin form VI.
Example 4: Preparation of fidaxomicin Form V:
Fidaxomicin
(500 mg) and a mixture of R-propylene glycol (5 mL) and water (15 mL)
were charged in Easy max reactor (Mettler Toledo). The reactor was set
to temperature cycle with following parameters:
Starting temperature is 25 °C;
Temperature raised to 60 °C over a period of 2 hours;
Cooled to 0 °C over a period of 2 hours;
Temperature raised to 60 °C over a period of 2 hours;
Cooled to 0 °C over a period of 2 hours;
Temperature raised to 25 °C over a period of 2 hours;
Temperature maintained at 25 °C for 2 hours.
After
completion of temperature cycling process, the slurry was filtered and
dried at 25°C to produce crystalline fidaxomicin form-V.
Example 5: Preparation of fidaxomicin Form V:
Fidaxomicin
(1 g) and a mixture of S-propylene glycol (3 ml_) and water (30 mL)
were charged in Easy max reactor (Mettler Toledo). The reactor was set
to temperature cycle with following parameters:
Starting temperature is 25 °C;
Temperature raised to 60 °C over a period of 2 hours;
Cooled to 0 °C over a period of 2 hours;
Temperature raised to 60 °C over a period of 2 hours;
Cooled to 0 °C over a period of 2 hours;
Temperature raised to 25 °C over a period of 2 hours;
Temperature maintained at 25 °C for 2 hours.
After
completion of temperature cycling process, the slurry was filtered and
dried at 25°C to produce crystalline fidaxomicin form-V.
Example 6: Preparation of fidaxomicin Form V:
Fidaxomicin
(40 g) and a mixture of propylene glycol (400 mL) and water (1600 mL)
were charged in Chem glass reactor. The reactor was set to temperature
cycle with following parameters:
Starting temperature is 25 °C;
Temperature raised to 60 °C over a period of 2 hours;
Cooled to 0 °C over a period of 2 hours;
Temperature raised to 60 °C over a period of 2 hours;
Cooled to 0 °C over a period of 2 hours;
Temperature raised to 25 °C over a period of 2 hours;
Temperature maintained at 25 °C for 6 hours.
After
completion of temperature cycling process, the slurry was filtered
under suction, followed by drying in air tray dryer (ATD) at 40°C to a
constant weight to produce crystalline fidaxomicin form-V.

The
10-member board at pharmaceutical major Dr Reddy’s thrives on
diversity. Liberally sprinkled with gray hairs, who are never quite
impressed with powerpoint presentations, “they want information to be
pre-loaded so that the following discussions (at the board level) are
fruitful,” says Satish Reddy, Chairman, Dr Reddy’s. That said, the
company has now equipped its board members with a customized application
(that runs on their tablets) to manage board agenda and related
processes.
see at
http://articles.economictimes.indiatimes.com/2014-10-31/news/55631761_1_board-members-board-agenda-dr-reddy-s

Dr. Reddy’s Laboratories Managing Director and Chief Operating Officer Satish Reddy addressing
References
- 1 "DIFICID" (PDF). TGA eBusiness Services. Specialised Therapeutics Australia Pty Ltd. 23 April 2013. Retrieved 31 March 2014.
- 2 Revill, P.; Serradell, N.; Bolós, J. (2006). "Tiacumicin B". Drugs of the Future 31 (6): 494. doi:10.1358/dof.2006.031.06.1000709.
- 3"Dificid, Full Prescribing Information" (PDF). Optimer Pharmaceuticals. 2013.
- 4 "Fidaxomicin". Drugs in R&D 10: 37. 2012. doi:10.2165/11537730-000000000-00000.
- 5Louie, T. J.; Emery, J.; Krulicki, W.; Byrne, B.; Mah, M. (2008). "OPT-80 Eliminates Clostridium difficile and is Sparing of Bacteroides Species during Treatment of C. Difficile Infection". Antimicrobial Agents and Chemotherapy 53 (1): 261–3. doi:10.1128/AAC.01443-07. PMC 2612159. PMID 18955523.
- 6Johnson, Stuart (2009). "Recurrent Clostridium difficile infection: A review of risk factors, treatments, and outcomes". Journal of Infection 58 (6): 403–10. doi:10.1016/j.jinf.2009.03.010. PMID 19394704.
- 7http://www.medicinescomplete.com/mc/bnf/current/PHP18388-dificlir.htm#PHP18388-dificlir
- 8Srivastava,
Aashish; Talaue, Meliza; Liu, Shuang; Degen, David; Ebright, Richard Y;
Sineva, Elena; Chakraborty, Anirban; Druzhinin, Sergey Y; Chatterjee,
Sujoy; Mukhopadhyay, Jayanta; Ebright, Yon W; Zozula, Alex; Shen, Juan;
Sengupta, Sonali; Niedfeldt, Rui Rong; Xin, Cai; Kaneko, Takushi;
Irschik, Herbert; Jansen, Rolf; Donadio, Stefano; Connell, Nancy; Ebright, Richard H (2011). "New target for inhibition of bacterial RNA polymerase: 'switch region'". Current Opinion in Microbiology 14 (5): 532–43. doi:10.1016/j.mib.2011.07.030. PMC 3196380. PMID 21862392.
- 9"Optimer's North American phase 3 Fidaxomicin study results presented at the 49th ICAAC" (Press release). Optimer Pharmaceuticals. September 16, 2009. Retrieved May 7, 2013.
- 10"Optimer Pharmaceuticals Presents Results From Fidaxomicin Phase 3 Study for the Treatment" (Press release). Optimer Pharmaceuticals. May 17, 2009. Retrieved May 7, 2013.
- 11Golan Y, Mullane KM, Miller MA (September 12–15, 2009). Low recurrence rate among patients with C. difficile infection treated with fidaxomicin. 49th interscience conference on antimicrobial agents and chemotherapy. San Francisco.
- 12Gorbach S, Weiss K, Sears P; et al. (September 12–15, 2009). Safety of fidaxomicin versus vancomycin in treatment of Clostridium difficile infection. 49th interscience conference on antimicrobial agents and chemotherapy. San Francisco.
- 13Louie,
Thomas J.; Miller, Mark A.; Mullane, Kathleen M.; Weiss, Karl; Lentnek,
Arnold; Golan, Yoav; Gorbach, Sherwood; Sears, Pamela; Shue, Youe-Kong;
Opt-80-003 Clinical Study, Group (2011). "Fidaxomicin versus vancomycin
for Clostridium difficile infection". New England Journal of Medicine 364 (5): 422–31. doi:10.1056/NEJMoa0910812. PMID 21288078.
- 14Peterson, Molly (Apr 5, 2011). "Optimer wins FDA panel's backing for antibiotic fidaxomicin". Bloomberg.
- 15Nordqvist, Christian (27 May 2011). "Dificid (fidaxomicin) approved for Clostridium difficile-associated diarrhea". Medical News Today.
ARNONE A ET AL: "
STRUCTURE ELUCIDATION OF THE MACROCYCLIC ANTIBIOTIC LIPIARMYCIN",
JOURNAL OF THE CHEMICAL SOCIETY, PERKIN TRANSACTIONS 1, CHEMICAL
SOCIETY, LETCHWORTH; GB, 1 January 1987 (1987-01-01), pages 1353-1359,
XP000578201, ISSN: 0300-922X, DOI: 10.1039/P19870001353
Fidaxomicin
 |
| Systematic (IUPAC) name |
|---|
3-(((6-Deoxy-4-O-(3,5-dichloro-2-ethyl-4,6-dihydroxybenzoyl)-2-O-methyl-β-D-mannopyranosyl)oxy)-methyl)-12(R)-[(6-deoxy-5-C-methyl-4-O-(2-methyl-1-oxopropyl)-β-D-lyxo-hexopyranosyl)oxy]-11(S)-ethyl-8(S)-hydroxy-18(S)-(1(R)-hydroxyethyl)-9,13,15-trimethyloxacyclooctadeca-3,5,9,13,15-pentaene-2-one
|
| Clinical data |
|---|
| Trade names | Dificid, Dificlir |
|---|
| Licence data | US FDA:link |
|---|
Pregnancy
category |
- AU: B1
- US: B (No risk in non-human studies)
|
|---|
| Legal status |
|
|---|
Routes of
administration | Oral |
|---|
| Pharmacokinetic data |
|---|
| Bioavailability | Minimal systemic absorption[1] |
|---|
| Biological half-life | 11.7 ± 4.80 hours[1] |
|---|
| Excretion | Urine (<1%), faeces (92%)[1] |
|---|
| Identifiers |
|---|
| CAS Number | 873857-62-6  |
|---|
| ATC code | A07AA12 |
|---|
| PubChem | CID 11528171 |
|---|
| ChemSpider | 8209640  |
|---|
| UNII | Z5N076G8YQ  |
|---|
| KEGG | D09394  |
|---|
| ChEBI | CHEBI:68590  |
|---|
| ChEMBL | CHEMBL1255800  |
|---|
| Synonyms | Clostomicin B1, lipiarmicin, lipiarmycin, lipiarmycin A3, OPT 80, PAR 01, PAR 101, tiacumicin B |
|---|
| Chemical data |
|---|
| Formula | C52H74Cl2O18 |
|---|
| Molar mass | 1058.04 g/mol |
|---|
| US4918174 | 26 Sep 1986 | 17 Apr 1990 | Abbott Laboratories | Tiacumicin compounds |
| WO2009025439A1 * | 6 May 2008 | 26 Feb 2009 | Genotech Co Ltd | Method
of extraction and yield-up of tricyclo compounds by adding a solid
adsorbent resin as their carrier in fermentation medium |
| WO2014023616A1 * | 30 Jul 2013 | 13 Feb 2014 | Olon Spa | Procedure for the production of tiacumicin b |
| WO2014111254A1 | 14 Jan 2014 | 24 Jul 2014 | Astellas Pharma Europe Ltd | Composition of tiacumicin compounds |
| WO2015091851A1 | 18 Dec 2014 | 25 Jun 2015 | Xellia Pharmaceuticals Aps | Process for the preparation of tiacumicin |
| WO2015169451A1 | 11 May 2015 | 12 Nov 2015 | Astellas Pharma Europe Ltd | Treatment regimen tiacumicin compound |
| CN101128114B | 31 Jan 2005 | 28 Mar 2012 | 浩鼎生技公司 | 18-membered macrocycles and analogs thereof |
| CN102614207B * | 31 Jan 2005 | 13 Jan 2016 | 默克夏普&多梅有限公司 | 18元环大环化合物及其类似物 |
| EP1848273A1 * | 31 Jan 2005 | 31 Oct 2007 | Optimer Pharmaceuticals, Inc. | 18-membered macrocycles and analogs thereof |
| EP2070530A1 | 13 May 2005 | 17 Jun 2009 | Optimer Pharmaceuticals, Inc. | Treatment of diseases associated with the use of antibiotics |
| EP2125850A1 † | 22 Jan 2008 | 2 Dec 2009 | Optimer Pharmaceuticals, Inc. | Macrocyclic polymorphs, compositions comprising such polymorphs, and methods of use and manufacture thereof |
| EP2305244A1 | 13 May 2005 | 6 Apr 2011 | Optimer Pharmaceuticals, Inc. | Treatment of diseases associated with the use of antibiotics |
| EP2305245A1 | 13 May 2005 | 6 Apr 2011 | Optimer Pharmaceuticals, Inc. | Treatment of diseases associated with the use of antibiotics |
| EP2468761A1 | 22 Jan 2008 | 27 Jun 2012 | Optimer Pharmaceuticals, Inc. | Macrocyclic polymorphs, compositions comprising such polymorphs, and methods of use and manufacture thereof |
| US7378508 | 31 Jul 2007 | 27 May 2008 | Optimer Pharmaceuticals, Inc. | Polymorphic crystalline forms of tiacumicin B |
| US7863249 | 11 Apr 2008 | 4 Jan 2011 | Optimer Pharmaceuticals, Inc. | Macrolide polymorphs, compositions comprising such polymorphs, and methods of use and manufacture thereof |
| US7906489 | 31 Jul 2007 | 15 Mar 2011 | Optimer Pharmaceuticals, Inc. | 18-membered macrocycles and analogs thereof |
| US8044030 | 28 Nov 2008 | 25 Oct 2011 | Optimer Pharmaceuticals, Inc. | Antibiotic macrocycle compounds and methods of manufacture and use thereof |
| US8586551 | 31 Aug 2009 | 19 Nov 2013 | Optimer Pharmaceuticals, Inc. | 18-membered macrocycles and analogs thereof |
| US8859510 | 22 Jan 2008 | 14 Oct 2014 | Optimer Pharmaceuticals, Inc. | Macrocyclic polymorphs, compositions comprising such polymorphs, and methods of use and manufacture thereof |
| US8883986 | 4 Mar 2009 | 11 Nov 2014 | Optimer Pharmaceuticals, Inc. | Macrolide polymorphs, compositions comprising such polymorphs, and methods of use and manufacture thereof |
| US8916527 | 15 Mar 2013 | 23 Dec 2014 | Optimer Pharmaceuticals, Inc. | Antibiotic macrocycle compounds and methods of manufacture and use thereof |
| US20110166090 * | | 7 Jul 2011 | Youe-Kong Shue | 18-Membered Macrocycles and Analogs Thereof |
| US20140107054 * | 21 Dec 2012 | 17 Apr 2014 | Optimer Pharmaceuticals, Inc. | Method of treating clostridium difficile-associated diarrhea |
| US3978211 * | Oct 31, 1974 | Aug 31, 1976 | Gruppo Lepetit S.P.A. | Lipiarmycin and its preparation |
| US4918174 | Sep 26, 1986 | Apr 17, 1990 | Abbott Laboratories | Tiacumicin compounds |
| US5583115 | May 9, 1995 | Dec 10, 1996 | Abbott Laboratories | Dialkyltiacumicin compounds |
| US5767096 | Jul 12, 1996 | Jun 16, 1998 | Abbott Laboratories | Bromotiacumicin compounds |
| US20060257981 * | Jul 15, 2003 | Nov 16, 2006 | Optimer Pharmaceuticals, Inc. | Tiacumicin production |
| US20070173462 * | May 13, 2005 | Jul 26, 2007 | Optimer Pharmaceuticals, Inc. | Treatment of diseases associated with the use of antibiotics |
| WO2004014295A2 | Jul 15, 2003 | Feb 19, 2004 | Optimer Pharmaceuticals Inc | Tiacumicin production |
| WO2005112990A2 | May 13, 2005 | Dec 1, 2005 | Optimer Pharmaceuticals Inc | Treatment of diseases associated with the use of antibiotics |
| WO2006085838A1 * | Jan 31, 2005 | Aug 17, 2006 | Optimer Pharmaceuticals Inc | 18-membered macrocycles and analogs thereof |
| DE2455230A1 * | Nov 21, 1974 | May 28, 1975 | Lepetit Spa | Lipiarmycin, verfahren zu seiner herstellung, mikroorganismus zur durchfuehrung des verfahrens und arzneimittel |
| EP2125850A1 | Jan 22, 2008 | Dec 2, 2009 | Optimer Pharmaceuticals, Inc. | Macrocyclic polymorphs, compositions comprising such polymorphs, and methods of use and manufacture thereof |
| US7378508 | Jul 31, 2007 | May 27, 2008 | Optimer Pharmaceuticals, Inc. | Polymorphic crystalline forms of tiacumicin B |
| Braga et al., "Making crystals from crystals: a green route tocrystal engineering and polymorphism" Chemical Communications (2005) pp. 3635-3645. |
| 2 | * | Chemical Abstracts registry entry 56645-60-4, Tiacumicin B, Copyright 2007, American Chemical Society, p. 1-2. |
| 3 | * | Dean, J., Analytical Chemistry Handbook, Published bt McGraw-Hill, Inc., pp. 10.23-10.26. |
| 4 | | J.E.
Hochlowski et al., Tiacumicins, A Novel Complex of 18-Membered
Macrolides, J. Antibiotics, vol. XL, No. 5, pp. 575-588 (May 1987). |
| 5 | * | Jain et al., "Polymorphism in Pharmacy" Indian Drugs (1986) vol. 23, No. 6, pp. 315-329. |
| 6 | * | Pharmaceutical
Dosage Forms: Tablets, vol. 2, Published by Marcel Dekker, Inc., ed. by
Lieberman, Lachman, and Schwartz, pp. 462-472. |
| 7 | * | Polymorphism in Pharmaceutical Solids, published 1999 by Marcel Dekker Inc, ed. by Harry G. Brittain, pp. 1-2. |
| 8 | | Robert
N. Swanson et al., In Vitro and In Vivo Evaluation of Tiacumicins B and
C against Clostridium difficile, Antimicrob. Agents Chemother., Jun.
1991, pp. 1108-1111. |
| 9 | * | The
Condensed Chemical Dictionary, Tenth Edition, published 1981 by the Van
Nostrand Reinhold Company, revised by Gessner G. Hawley, p. 35 and 835. |
///////////Fidaxomicin, OPT-80, PAR-101
CC[C@H]1/C=C(/[C@H](C/C=C/C=C(/C(=O)O[C@@H](C/C=C(/C=C(/[C@@H]1O[C@H]2[C@H]([C@H]([C@@H](C(O2)(C)C)OC(=O)C(C)C)O)O)\C)\C)[C@@H](C)O)\CO[C@H]3[C@H]([C@H]([C@@H]([C@H](O3)C)OC(=O)C4=C(C(=C(C(=C4O)Cl)O)Cl)CC)O)OC)O)\C