DRUG SYNTHESIS « New Drug Approvals:
'via Blog this'
Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, helping millions with websites, 9 million hits on google, 2.5 lakh connections worldwide, P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
Thursday, 31 October 2013
Tuesday, 29 October 2013
Bristol-Myers Squibb announced promising results from an expanded phase 1 dose-ranging study of its lung cancer drug nivolumab « New Drug Approvals
Monday, 28 October 2013
Sunday, 27 October 2013
FDA In India: Going Global, Coming Home, Altaf Ahmed Lal, Ph.D. is the Director of FDA’s office in India.

Altaf Ahmed Lal, Ph.D. is the Director of FDA’s office in India.
By: Altaf Ahmed Lal, Ph.D.
What is it like to be starting my new position as director of FDA’s office in India?
It’s like coming home.
My new tenure at FDA began in June, but as a former health attaché in the U.S. Embassy, I played an enthusiastic role in helping to establish FDA in my native country. I was born in Kashmir, India, and though I left the country in 1980 to explore new professional opportunities in the United States, I have since been drawn back again and again.
Saturday, 26 October 2013
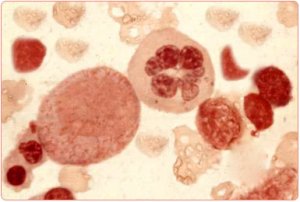
Aplastic Anemia

Aplastic anemia is a rare and potentially serious blood disorder in which the body's bone marrow doesn't make enough red blood cells, white blood cells, and platelets. In many people who have aplastic anemia, the cause is unknown but may be due to one of a number of causes (eg, toxins, cancer treatment, some medicines, infections, autoimmune disorders, pregnancy). Treatments for aplastic anemia include blood transfusions, blood and marrow stem cell transplants, and medicines to stimulate bone marrow and to prevent infections. At present, there are no orphan drugs approved for aplastic anemia. For more information about aplastic anemia, visit http://www.nhlbi.nih.gov/health/health-topics/topics/aplastic/

Aplastic anemia is a syndrome of bone marrow failure characterized by peripheral pancytopenia and marrow hypoplasia, and mild macrocytosis is observed in association with stress erythropoiesis and an elevated fetal hemoglobin levels. Paul Ehrlich introduced the concept of aplastic anemia in 1888 when he studied the case of a pregnant woman who died of bone marrow failure. However, it was not until 1904 that Anatole Chauffard named this disorder aplastic anemia.
For excellent patient education resources, visit eMedicine‘s Blood and Lymphatic System Center. Also, see eMedicine’s patient education article Anemia.
Medication
The goals of pharmacotherapy in cases of aplastic anemia are to reduce morbidity, prevent complications, and eradicate malignancy.
Immunosuppressive Agents
The merits of additional immunosuppression versus the increased risk and cost should be considered. Data from a randomized prospective study indicated that an increased proportion of patients responded to the addition of CSA to ATG, but this did not translate into a long-term survival advantage.
For patients who cannot tolerate equine-based products, use of the commercially available rabbit-based ATG product (Thymoglobulin) may be considered. This product is currently approved in the United States and has been used for the treatment of aplastic anemia in Europe (although note the different dose schedule).
Cyclosporine (Sandimmune, Neoral)
Cyclic polypeptide that suppresses some humoral immunity and, to a greater extent, cell-mediated immune reactions (eg, delayed hypersensitivity, allograft rejection, experimental allergic encephalomyelitis, and graft vs host disease) for a variety of organs.
For children and adults, base the dosing on the ideal body weight. Frequent monitoring of drug levels is needed. To convert to the PO dose, use a IV-to-PO correction factor of 1:4. Dosage and duration of therapy may vary with different protocols.
- Dosing
- Interactions
- Contraindications
- Precautions
Adult
1.5-2 mg/kg IV q12h, adjust to trough level of 500-800 ng/mL in mo 1 or so; then adjust to trough level of 200 ng/mL
Pediatric
Administer as in adults.
Methylprednisolone (Medrol, Solu-Medrol)
Steroids ameliorate the delayed effects of anaphylactoid reactions and may limit biphasic anaphylaxis. In severe serum sickness (mediated by immune complexes), parenteral steroids may reduce the inflammatory effects. Hence, used with ATG to decrease the adverse effects (eg, allergic reactions, serum sickness). Also an additional immunosuppressive. High doses or long duration may be needed if serum sickness occurs with ATG. The doses and duration may vary with different protocols.
- Dosing
- Interactions
- Contraindications
- Precautions
Adult
5 mg/kg IV on days 1-8; then tapered by using PO 1 mg/kg on days 9-14; further tapering over days 15-29; stop after 1 mo except with evidence of serum sickness
Pediatric
Administer as in adults.
Lymphocyte immune globulin, equine (Atgam)
Inhibits cell-mediated immune response by altering T-cell function or eliminating antigen-reactive cells.There is little prospective randomized data to suggest a single schedule superior, but experience suggests that a short infusion is best tolerated.
- Dosing
- Interactions
- Contraindications
- Precautions
Adult
100-200 mg/kg IV total dose over variable number of days based on different protocols
Pediatric
Administer as in adults.
Cyclophosphamide (Cytoxan)
Chemically related to nitrogen mustards. As an alkylating agent, the mechanism of action of the active metabolites may involve cross-linking of DNA, which may interfere with the growth of normal and neoplastic cells. Monitor carefully; used only on an investigational basis.
- Dosing
- Interactions
- Contraindications
- Precautions
Adult
45 mg/kg/d IV for 4 d
Pediatric
Administer as in adults
Lymphocyte immune globulin, rabbit (Thymoglobulin)
May modify T-cell function and possibly eliminate antigen-reactive T lymphocytes in peripheral blood. The dose and duration of therapy vary with the investigational protocols.
- Dosing
- Interactions
- Contraindications
- Precautions
Adult
1.5 mg/kg IV qd for 7-14 d; up to 3.5 mg/kg for 5 d also used
Pediatric
Not established
Cytokines
Several preliminary studies have demonstrated that the addition of cytokines (eg, G-CSF, GM-CSF) may hasten the neutrophil recovery and that these agents may improve the response rate and survival, although long-term use may increase the risk of clonal evolution.
Sargramostim (Leukine, Prokine)
Recombinant human GM-CSF. Can activate mature granulocytes and macrophages. The dose and frequency of administration vary with the investigational protocol.
- Dosing
- Interactions
- Contraindications
- Precautions
Adult
250 mcg/m2 IV/SC with twice weekly monitoring of CBC count
Pediatric
Not established; 5 mcg/kg/d SC used in some studies
Filgrastim (Neupogen)
G-CSF that activates and stimulates the production, maturation, migration, and cytotoxicity of neutrophils.
- Dosing
- Interactions
- Contraindications
- Precautions
Adult
5 mcg/kg/d SC until ANC 5000/mm3
Pediatric
5-10 mcg/kg/d SC
Antineoplastic Agent, Antimetabolite (purine)
Antimetabolites are antineoplastic agent that inhibit cell growth and proliferation.
Fludarabine (Fludara)
Contains fludarabine phosphate, a fluorinated nucleotide analogue of the antiviral agent vidarabine, 9-b-D-arabinofuranosyladenine (ara-A) that enters the cell and is phosphorylated to form active metabolite 2-fluoro-ara-ATP, which inhibits DNA synthesis. Inhibits DNA polymerase, DNA primase, DNA ligase, and ribonucleotide reductase. This inhibits RNA function, RNA processing, and mRNA translation. Also activates apoptosis.
- Dosing
- Interactions
- Contraindications
- Precautions
Adult
30 mg/m2/dose for 4-6 d as IV infusion over 30 min-2 h
Pediatric
Administer as in adults
The theoretical basis for marrow failure includes primary defects in or damage to the stem cell or the marrow microenvironment.1,2,3 The distinction between acquired and inherited disease may present a clinical challenge, but more than 80% of cases are acquired. In acquired aplastic anemia, clinical and laboratory observations suggest that this is an autoimmune disease.
On morphologic evaluation, the bone marrow is devoid of hematopoietic elements, showing largely fat cells. Flow cytometry shows that the CD34 cell population, which contains the stem cells and the early committed progenitors, is substantially reduced.2,4 Data from in vitro colony-culture assays suggest profound functional loss of the hematopoietic progenitors, so much so that they are unresponsive even to high levels of hematopoietic growth factors.
Little evidence points to a defective microenvironment as a cause of aplastic anemia. In patients with severe aplastic anemia (SAA), stromal cells have normal function, including growth factor production. Adequate stromal function is implicit in the success of bone marrow transplantation (BMT) in aplastic anemia because the stromal elements are frequently of host origin.
The role of an immune dysfunction was suggested in 1970, when autologous recovery was documented in a patient with aplastic anemia in whom engrafting failed after BMT. Mathe proposed that the immunosuppressive regimen used for conditioning promoted the return of normal marrow function. Since then, numerous studies have shown that, in approximately 70% of patients with acquired aplastic anemia, immunosuppressive therapy improves marrow function.3,5,6,7,8 Immunity is genetically regulated (by immune response genes), and it is also influenced by environment (eg, nutrition, aging, previous exposure).9,10 Although the inciting antigens that breach immune tolerance with subsequent autoimmunity are unknown, human leukocyte antigen (HLA)-DR2 is overrepresented among European and United States patients with aplastic anemia, suggesting a role for antigen recognition, and its presence is predictive of a better response to cyclosporine.
Suppression of hematopoiesis is likely mediated by an expanded population of the following cytotoxic T lymphocytes (CTLs): CD8 and HLA-DR+, which are detectable in both the blood and bone marrow of patients with aplastic anemia. These cells produce inhibitory cytokines, such as gamma-interferon and tumor necrosis factor, which can suppress progenitor cell growth. Polymorphisms in these cytokine genes, associated with an increased immune response, are more prevalent in patients with aplastic anemia. These cytokines suppress hematopoiesis by affecting the mitotic cycle and cell killing by inducing Fas-mediated apoptosis. In addition, these cytokines induce nitric oxide synthase and nitric oxide production by marrow cells, which contributes to immune-mediated cytotoxicity and the elimination of hematopoietic cells.
Constitutive expression of Tbet, a transcriptional regulator that is critical to Th1 polarization, occurs in a majority of aplastic anemia patients.5 Perforin is a cytolytic protein expressed mainly in activated cytotoxic lymphocytes and natural-killer cells. Mutations in perforin gene are responsible for some cases of familial hemophagocytosis11 ; mutations in SAP, a gene encoding for a small modulator protein that inhibits undefined-interferon production, underlie X-linked lymphoproliferation, a fatal illness associated with an aberrant immune response to herpesviruses and aplastic anemia. Perforin and SAP protein levels are markedly diminished in a majority of acquired aplastic anemia cases.
Friday, 25 October 2013
Thursday, 24 October 2013
Cubist Pharmaceuticals, Inc. announced that it has submitted a NDA to the U.S. FDA for approval of its investigational antibiotic tedizolid phosphate (TR-701). « New Drug Approvals
Why Antibody Lots Differ Significantly
http://www.dddmag.com/news/2013/10/why-antibody-lots-differ-significantly?et_cid=3555713&et_rid=523035093&type=cta
fig is Schematic representation of pure and contaminated antibodies carrying histones or histones complexed with DNA. (Source: Biochemistry and Cell Biology)
fig is Schematic representation of pure and contaminated antibodies carrying histones or histones complexed with DNA. (Source: Biochemistry and Cell Biology)
Tuesday, 22 October 2013
RIOCIQUAT
October 8, 2013 — The U.S. Food and Drug Administration today approved Adempas (riociguat) to treat adults with two forms of pulmonary hypertension.
Pulmonary hypertension is caused by abnormally high blood pressure in the arteries of the lungs. It makes the right side of the heart work harder than normal. In its various forms, pulmonary hypertension is a chronic, progressive, debilitating disease, often leading to death or need for lung transplantation
read all at
In the area of pulmonary hypertension Adempas (Riociguat) is the first member of a novel class of compounds – so-called ‘soluble guanylate cyclase (sGC) stimulators’ – being investigated as a new and specific approach to treating different types of pulmonary hypertension (PH). Adempas has the potential to overcome a number of limitations of currently approved treatments for pulmonary arterial hypertension (PAH) and addresses the unmet medical need in patients with chronic thromboembolic pulmonary hypertension (CTEPH). It was approved for the treatment of CTEPH in Canada in September 2013, making it the world’s first drug approved in this deadly disease.
Riociguat has already shown promise as a potential treatment option beyond these two PH indications. An early clinical study was conducted in PH-ILD (interstitial lung disease), a disease characterized by lung tissue scarring (fibrosis) or lung inflammation which can lead to pulmonary hypertension, and, based on positive data, the decision was taken to initiate Phase IIb studies in PH-IIP (idiopathic pulmonary fibrosis), a subgroup of PH-ILD. Moreover, scientific evidence was demonstrated in preclinical models that the activity may even go beyond vascular relaxation. To prove the hypothesis Bayer is initiating clinical studies in the indication of systemic sclerosis (SSc), an orphan chronic autoimmune disease of the connective tissue affecting several organs and associated with high morbidity and mortality. If successful, Riociguat has the potential to become the first approved treatment for this devastating disease.
Riociguat has already shown promise as a potential treatment option beyond these two PH indications. An early clinical study was conducted in PH-ILD (interstitial lung disease), a disease characterized by lung tissue scarring (fibrosis) or lung inflammation which can lead to pulmonary hypertension, and, based on positive data, the decision was taken to initiate Phase IIb studies in PH-IIP (idiopathic pulmonary fibrosis), a subgroup of PH-ILD. Moreover, scientific evidence was demonstrated in preclinical models that the activity may even go beyond vascular relaxation. To prove the hypothesis Bayer is initiating clinical studies in the indication of systemic sclerosis (SSc), an orphan chronic autoimmune disease of the connective tissue affecting several organs and associated with high morbidity and mortality. If successful, Riociguat has the potential to become the first approved treatment for this devastating disease.
synthesis
Generic Name: Riociguat
Trade Name: Adempas
Synonym: BAY 63-2521
CAS number: 625115-55-1
Chemical Name: Methyl N-[4,6-Diamino-2-[1-[(2-fluorophenyl)methyl]-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-pyrimidinyl]-N-methyl-carbaminate
Mechanism of Action: soluble guanylyl cyclase (sGC) stimulator
Date of Approval: October 8, 2013(US)
Indication: Pulmonary Hypertension
Company: Bayer AG
Trade Name: Adempas
Synonym: BAY 63-2521
CAS number: 625115-55-1
Chemical Name: Methyl N-[4,6-Diamino-2-[1-[(2-fluorophenyl)methyl]-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-pyrimidinyl]-N-methyl-carbaminate
Mechanism of Action: soluble guanylyl cyclase (sGC) stimulator
Date of Approval: October 8, 2013(US)
Indication: Pulmonary Hypertension
Company: Bayer AG

1)J. Mittendorf.; S. Weigand.; C. Alonso-Alija.; E. Bischoff.; A. Feurer.; M. Gerisch.; A. Kern.; A. Knorr.; D. Lang.; K. Muenter.; M. Radtke.; H. Schirok.; K.-H. Schlemmer.; E. Stahl.; A. Straub.; F. Wunder.; J.-P. Stasch. Discovery of Riociguat (BAY 63-2521): A Potent, Oral Stimulator of Soluble Guanylate Cyclase for the Treatment of Pulmonary Hypertension, ChemMedChem. 2009, 4, 853-865.
2)Cristina Alonso-Alija, Bayer Ag, Erwin Bischoff, Achim Feurer, Klaus Muenter, Elke Stahl, Johannes-Peter Stasch, Stefan Weigand, Carbamate-substituted pyrazolopyridines, WO2003095451 A1
3)Franz-Josef Mais, Joachim Rehse, Winfried Joentgen, Konrad SIEGEL, Process for preparing methyl methylcarbamate and its purification for use as pharmaceutically active compound,US20110130410
4)Claudia Hirth-Dietrich, Peter Sandner, Johannes-Peter Stasch, Andreas Knorr, Degenfeld Georges Von, Michael Hahn, Markus Follmann, The use of sGC stimulators, sGC activators, alone and combinations with PDE5 inhibitors for the treatment of systemic sclerosis (SSc), WO 2011147810A1
5)Li Liang, Li Xing-zhou, Liu Ya-dan, Zheng Zhi-bing, Li Song, Synthesis of riociguat in treatment of pulmonary hypertension, Chinese Journal of Medicinal Chemistry(Zhongguo Yaowu Huaxue Zazhi), 21(2),120-125; 2011
2)Cristina Alonso-Alija, Bayer Ag, Erwin Bischoff, Achim Feurer, Klaus Muenter, Elke Stahl, Johannes-Peter Stasch, Stefan Weigand, Carbamate-substituted pyrazolopyridines, WO2003095451 A1
3)Franz-Josef Mais, Joachim Rehse, Winfried Joentgen, Konrad SIEGEL, Process for preparing methyl methylcarbamate and its purification for use as pharmaceutically active compound,US20110130410
4)Claudia Hirth-Dietrich, Peter Sandner, Johannes-Peter Stasch, Andreas Knorr, Degenfeld Georges Von, Michael Hahn, Markus Follmann, The use of sGC stimulators, sGC activators, alone and combinations with PDE5 inhibitors for the treatment of systemic sclerosis (SSc), WO 2011147810A1
5)Li Liang, Li Xing-zhou, Liu Ya-dan, Zheng Zhi-bing, Li Song, Synthesis of riociguat in treatment of pulmonary hypertension, Chinese Journal of Medicinal Chemistry(Zhongguo Yaowu Huaxue Zazhi), 21(2),120-125; 2011

Jens Ackerstaff, Lars BÄRFACKER, Markus Follmann, Nils Griebenow, Andreas Knorr, Volkhart Min-Jian Li, Gorden Redlich, Johannes-Peter Stasch, Stefan Weigand, Frank Wunder, Bicyclic aza heterocycles, and use thereof, WO2012028647 A1
2)Claudia Hirth-Dietrich, Peter Sandner, Johannes-Peter Stasch, Andreas Knorr, Degenfeld Georges Von, Michael Hahn, Markus Follmann, The use of sGC stimulators, sGC activators, alone and combinations with PDE5 inhibitors for the treatment of systemic sclerosis (SSc), WO 2011147810A1
2)Claudia Hirth-Dietrich, Peter Sandner, Johannes-Peter Stasch, Andreas Knorr, Degenfeld Georges Von, Michael Hahn, Markus Follmann, The use of sGC stimulators, sGC activators, alone and combinations with PDE5 inhibitors for the treatment of systemic sclerosis (SSc), WO 2011147810A1

Jin Li, Xiaoyu Yang, Jingwei ZHU, Minmin Yang, Xihan Wu, Method for synthesizing 1-(2-fluorobenzyl)-1H -pyrazolo[3,4-b]pyridin -3-formamidine hydrochloride, WO2013086935 A1

veerareddy Arava, Surendrareddy Gogireddy, An expeditious synthesis of riociguat, A pulmonary hypertension drug, Der Pharma Chemica, 2013, 5(4):232-239
cut paste from my earlier post
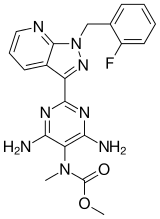
RIOCIQUAT
CAS NO 625115-55-1
Methyl N-[4,6-Diamino-2-[1-[(2-fluorophenyl)methyl]-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-pyrimidinyl]-N-methyl-carbaminate
9 APRIL2013
Bayer has been boosted by the news that regulators in the USA are fast-tracking the German group’s investigational pulmonary arterial hypertension riociguat.
The US Food and Drug Administration has granted priority review to the New Drug Application for riociguat, which Bayer filed in February on both sides of the Atlantic for PAH and a related condition, inoperable chronic thromboembolic pulmonary hypertension (CTEPH). The FDA bestows a priority review on medicines that offer major advances in care or that provide a treatment where no adequate therapy exists. The agency aims to complete its assessment within eight months from the submission of the NDA, rather than the standard 12 months.
Riociguat (BAY 63-2521) is a novel drug that is currently in clinical development by Bayer. It is a stimulator of soluble guanylate cyclase (sGC). At the moment Phase III clinical trialsinvestigate the use of riociguat as a new approach to treat two forms of pulmonary hypertension (PH): chronic thromboembolic pulmonary hypertension (CTEPH) andpulmonary arterial hypertension (PAH). Riociguat constitutes the first drug of a novel class of sGC stimulators
The submissions are based on two Phase III studies and riociguat, the first member of a novel class of compounds called stimulators of soluble guanylate cyclase (sGC), met its primary endpoint in both trials, a change in exercise capacity after 12- or 16 weeks respectively. The drug was generally well tolerated, with a good safety profile.
Kemal Malik, head of global development at Bayer Healthcare, noted that “to date, no approved pharmacological therapy exists for CTEPH and as a result there is an urgent unmet medical need for patients who are unable to undergo surgery. We hope that we will soon be in a position to provide patients and doctors with a new treatment option”.

If approved, riociguat would be going up against Actelion’s Tracleer (bosentan) and Gilead Sciences/GlaxoSmithKline’s Letairis/Volibris (ambrisentan). Actelion, which has dominated the PAH market, has already filed its follow-up to Tracleer, Opsumit (macitentan).
Macitentan

MACITENTAN
N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N’-propylsulfamide,
CAS NO 441798-33-0
Macitentan (Opsumit® )is a novel dual endothelin receptor antagonist that resulted from a tailored drug discovery process. Macitentan has a number of potentially key beneficial characteristics – i.e., increased in vivo preclinical efficacy vs. existing ERAs resulting from sustained receptor binding and tissue penetration properties. A clinical pharmacology program indicated a low propensity of macitentan for drug-drug interactions.
Macitentan is an investigational drug being studied for the treatment of pulmonary arterial hypertension. It acts as a dualendothelin receptor antagonist and is being developed by Actelion.[1] A Phase III clinical trial was successfully completed in 2012.[2]
on 22 October 2012 - Actelion (SIX: ATLN) announced that it has submitted a New Drug Application (NDA) to the US Food and Drug Administration (FDA) seeking approval for macitentan (Opsumit®) for the treatment of patients with pulmonary arterial hypertension
Actelion’s experimental lung drug macitentan prolonged overall survival by more than a third according to detailed study data, which the company hopes will convince investors it has a viable follow-up product to secure its commercial future.
Europe’s largest standalone biotech company wants the drug, which treats pulmonary arterial hypertension — a disease that causes high blood pressure in the arteries of the lungs — to replace blockbuster Tracleer.
Tracleer currently makes up 87 percent of sales but loses patent protection in 2015 and has also seen its market share eroded by Gilead’s Letairis.
Pharmacokinetics
Macitentan has an active metabolite, ACT-132577, which is an oxidative depropylation product. Both macitentan and ACT-132577 are mainly excreted in form of hydrolysis products via urine (about 2/3 of all metabolites) and faeces (1/3).[3]
Co-administration of ciclosporin has only a slight effect on the concentrations of macitentan and its active metabolite, whilerifampicin decreases the area under the curve (AUC) of the drug’s blood plasma concentration by 79%, and ketoconazoleapproximately doubles it. This corresponds to the finding that macitentan is mainly metabolised via the liver enzyme CYP3A4.[4]
- ^ Bolli, M. H.; Boss, C.; Binkert, C.; Buchmann, S.; Bur, D.; Hess, P.; Iglarz, M.; Meyer, S.; Rein, J.; Rey, M.; Treiber, A.; Clozel, M.; Fischli, W.; Weller, T. (2012). “The Discovery of N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N′-propylsulfamide (Macitentan), an Orally Active, Potent Dual Endothelin Receptor Antagonist”. Journal of Medicinal Chemistry 55 (17): 7849–7861. doi:10.1021/jm3009103. PMID 22862294. edit
- ^ “Macitentan”. Actelion. Retrieved 22 August 2012.
- ^ Bruderer, S.; Hopfgartner, G. R.; Seiberling, M.; Wank, J.; Sidharta, P. N.; Treiber, A.; Dingemanse, J. (2012). “Absorption, distribution, metabolism, and excretion of macitentan, a dual endothelin receptor antagonist, in humans”. Xenobiotica 42 (9): 901–910.doi:10.3109/00498254.2012.664665. PMID 22458347. edit
- ^ Bruderer, S.; Äänismaa, P. I.; Homery, M. C.; Häusler, S.; Landskroner, K.; Sidharta, P. N.; Treiber, A.; Dingemanse, J. (2011).“Effect of Cyclosporine and Rifampin on the Pharmacokinetics of Macitentan, a Tissue-Targeting Dual Endothelin Receptor Antagonist”. The AAPS Journal 14 (1): 68–78. doi:10.1208/s12248-011-9316-3. PMC 3282010. PMID 22189899. edit
External links
Actelion Ltd
Actelion Ltd is a biopharmaceutical company with its corporate headquarters in Allschwil/Basel, Switzerland. Actelion’s first drug Tracleer®, an orally available dual endothelin receptor antagonist, has been approved as a therapy for pulmonary arterial hypertension. Actelion markets Tracleer through its own subsidiaries in key markets worldwide, including the United States (based in South San Francisco), the European Union, Japan, Canada, Australia and Switzerland. Actelion, founded in late 1997, is a leading player in innovative science related to the endothelium – the single layer of cells separating every blood vessel from the blood stream. Actelion’s over 2,400 employees focus on the discovery, development and marketing of innovative drugs for significant unmet medical needs. Actelion shares are traded on the SIX Swiss Exchange (ticker symbol: ATLN) as part of the Swiss blue-chip index SMI (Swiss Market Index SMI®).
synthesis
Generic Name:Macitentan
Trade Name:Opsumit
Synonym:ACT-064992
Mechanism of Action:Endothelin receptor antagonist (ERA)
Date of Approval: October 18, 2013(US)
Indication: Pulmonary Hypertension (PAH)
Company: Actelion Pharmaceuticals Ltd
CAS number: 441798-33-0
ChemicalName: N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl] -N’-propylsulfamide
PCT patent application: WO2002053557
Trade Name:Opsumit
Synonym:ACT-064992
Mechanism of Action:Endothelin receptor antagonist (ERA)
Date of Approval: October 18, 2013(US)
Indication: Pulmonary Hypertension (PAH)
Company: Actelion Pharmaceuticals Ltd
CAS number: 441798-33-0
ChemicalName: N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl] -N’-propylsulfamide
PCT patent application: WO2002053557

…
1)Bolli, M. H.; Boss, C.; Binkert, C.; Buchmann, S.; Bur, D.; Hess, P.; Iglarz, M.; Meyer, S.; Rein, J.; Rey, M.; Treiber, A.; Clozel, M.; Fischli, W.; Weller, T. (2012). “The Discovery of N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N′-propylsulfamide (Macitentan), an Orally Active, Potent Dual Endothelin Receptor Antagonist“. Journal of Medicinal Chemistry, 2012, 55 (17): 7849–7861
2)Martin Bolli, Christoph Boss, Martine Clozel, Walter Fischli, Thomas Weller, Novel sulfamides and their use as endothelin receptor antagonists, WO2002053557 A1, CA2431675A1, CA2431675C, CN1524079A, CN100432070C, DE60118782D1, DE60118782T2, EP1345920A1, EP1345920B1, EP1693372A1, US7094781, US7285549, US20040077670, US20060178365,
3)Martin Bolli, Christoph Boss, Martine Clozel, Walter Fischli, Thomas Weller, Sulfamides as endothelin receptor antagonists for the treatment of cardiovascular diseases, WO2006051502
4)Martine Clozel, Therapeutic compositions containing macitentan,WO2010018549 A2, CA2731370A1, CN102099026A, CN102099026B, EP2315587A2, US20110136818
2)Martin Bolli, Christoph Boss, Martine Clozel, Walter Fischli, Thomas Weller, Novel sulfamides and their use as endothelin receptor antagonists, WO2002053557 A1, CA2431675A1, CA2431675C, CN1524079A, CN100432070C, DE60118782D1, DE60118782T2, EP1345920A1, EP1345920B1, EP1693372A1, US7094781, US7285549, US20040077670, US20060178365,
3)Martin Bolli, Christoph Boss, Martine Clozel, Walter Fischli, Thomas Weller, Sulfamides as endothelin receptor antagonists for the treatment of cardiovascular diseases, WO2006051502
4)Martine Clozel, Therapeutic compositions containing macitentan,WO2010018549 A2, CA2731370A1, CN102099026A, CN102099026B, EP2315587A2, US20110136818
Subscribe to:
Comments (Atom)