October 8, 2013 — The U.S. Food and Drug Administration today approved Adempas (riociguat) to treat adults with two forms of pulmonary hypertension.
Pulmonary hypertension is caused by abnormally high blood pressure in the arteries of the lungs. It makes the right side of the heart work harder than normal. In its various forms, pulmonary hypertension is a chronic, progressive, debilitating disease, often leading to death or need for lung transplantation
read all at
In the area of pulmonary hypertension Adempas (Riociguat) is the first member of a novel class of compounds – so-called ‘soluble guanylate cyclase (sGC) stimulators’ – being investigated as a new and specific approach to treating different types of pulmonary hypertension (PH). Adempas has the potential to overcome a number of limitations of currently approved treatments for pulmonary arterial hypertension (PAH) and addresses the unmet medical need in patients with chronic thromboembolic pulmonary hypertension (CTEPH). It was approved for the treatment of CTEPH in Canada in September 2013, making it the world’s first drug approved in this deadly disease.
Riociguat has already shown promise as a potential treatment option beyond these two PH indications. An early clinical study was conducted in PH-ILD (interstitial lung disease), a disease characterized by lung tissue scarring (fibrosis) or lung inflammation which can lead to pulmonary hypertension, and, based on positive data, the decision was taken to initiate Phase IIb studies in PH-IIP (idiopathic pulmonary fibrosis), a subgroup of PH-ILD. Moreover, scientific evidence was demonstrated in preclinical models that the activity may even go beyond vascular relaxation. To prove the hypothesis Bayer is initiating clinical studies in the indication of systemic sclerosis (SSc), an orphan chronic autoimmune disease of the connective tissue affecting several organs and associated with high morbidity and mortality. If successful, Riociguat has the potential to become the first approved treatment for this devastating disease.
Riociguat has already shown promise as a potential treatment option beyond these two PH indications. An early clinical study was conducted in PH-ILD (interstitial lung disease), a disease characterized by lung tissue scarring (fibrosis) or lung inflammation which can lead to pulmonary hypertension, and, based on positive data, the decision was taken to initiate Phase IIb studies in PH-IIP (idiopathic pulmonary fibrosis), a subgroup of PH-ILD. Moreover, scientific evidence was demonstrated in preclinical models that the activity may even go beyond vascular relaxation. To prove the hypothesis Bayer is initiating clinical studies in the indication of systemic sclerosis (SSc), an orphan chronic autoimmune disease of the connective tissue affecting several organs and associated with high morbidity and mortality. If successful, Riociguat has the potential to become the first approved treatment for this devastating disease.
synthesis
Generic Name: Riociguat
Trade Name: Adempas
Synonym: BAY 63-2521
CAS number: 625115-55-1
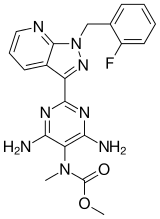
Chemical Name: Methyl N-[4,6-Diamino-2-[1-[(2-fluorophenyl)methyl]-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-pyrimidinyl]-N-methyl-carbaminate
Mechanism of Action: soluble guanylyl cyclase (sGC) stimulator
Date of Approval: October 8, 2013(US)
Indication: Pulmonary Hypertension
Company: Bayer AG
Trade Name: Adempas
Synonym: BAY 63-2521
CAS number: 625115-55-1
Chemical Name: Methyl N-[4,6-Diamino-2-[1-[(2-fluorophenyl)methyl]-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-pyrimidinyl]-N-methyl-carbaminate
Mechanism of Action: soluble guanylyl cyclase (sGC) stimulator
Date of Approval: October 8, 2013(US)
Indication: Pulmonary Hypertension
Company: Bayer AG

1)J. Mittendorf.; S. Weigand.; C. Alonso-Alija.; E. Bischoff.; A. Feurer.; M. Gerisch.; A. Kern.; A. Knorr.; D. Lang.; K. Muenter.; M. Radtke.; H. Schirok.; K.-H. Schlemmer.; E. Stahl.; A. Straub.; F. Wunder.; J.-P. Stasch. Discovery of Riociguat (BAY 63-2521): A Potent, Oral Stimulator of Soluble Guanylate Cyclase for the Treatment of Pulmonary Hypertension, ChemMedChem. 2009, 4, 853-865.
2)Cristina Alonso-Alija, Bayer Ag, Erwin Bischoff, Achim Feurer, Klaus Muenter, Elke Stahl, Johannes-Peter Stasch, Stefan Weigand, Carbamate-substituted pyrazolopyridines, WO2003095451 A1
3)Franz-Josef Mais, Joachim Rehse, Winfried Joentgen, Konrad SIEGEL, Process for preparing methyl methylcarbamate and its purification for use as pharmaceutically active compound,US20110130410
4)Claudia Hirth-Dietrich, Peter Sandner, Johannes-Peter Stasch, Andreas Knorr, Degenfeld Georges Von, Michael Hahn, Markus Follmann, The use of sGC stimulators, sGC activators, alone and combinations with PDE5 inhibitors for the treatment of systemic sclerosis (SSc), WO 2011147810A1
5)Li Liang, Li Xing-zhou, Liu Ya-dan, Zheng Zhi-bing, Li Song, Synthesis of riociguat in treatment of pulmonary hypertension, Chinese Journal of Medicinal Chemistry(Zhongguo Yaowu Huaxue Zazhi), 21(2),120-125; 2011
2)Cristina Alonso-Alija, Bayer Ag, Erwin Bischoff, Achim Feurer, Klaus Muenter, Elke Stahl, Johannes-Peter Stasch, Stefan Weigand, Carbamate-substituted pyrazolopyridines, WO2003095451 A1
3)Franz-Josef Mais, Joachim Rehse, Winfried Joentgen, Konrad SIEGEL, Process for preparing methyl methylcarbamate and its purification for use as pharmaceutically active compound,US20110130410
4)Claudia Hirth-Dietrich, Peter Sandner, Johannes-Peter Stasch, Andreas Knorr, Degenfeld Georges Von, Michael Hahn, Markus Follmann, The use of sGC stimulators, sGC activators, alone and combinations with PDE5 inhibitors for the treatment of systemic sclerosis (SSc), WO 2011147810A1
5)Li Liang, Li Xing-zhou, Liu Ya-dan, Zheng Zhi-bing, Li Song, Synthesis of riociguat in treatment of pulmonary hypertension, Chinese Journal of Medicinal Chemistry(Zhongguo Yaowu Huaxue Zazhi), 21(2),120-125; 2011

Jens Ackerstaff, Lars BÄRFACKER, Markus Follmann, Nils Griebenow, Andreas Knorr, Volkhart Min-Jian Li, Gorden Redlich, Johannes-Peter Stasch, Stefan Weigand, Frank Wunder, Bicyclic aza heterocycles, and use thereof, WO2012028647 A1
2)Claudia Hirth-Dietrich, Peter Sandner, Johannes-Peter Stasch, Andreas Knorr, Degenfeld Georges Von, Michael Hahn, Markus Follmann, The use of sGC stimulators, sGC activators, alone and combinations with PDE5 inhibitors for the treatment of systemic sclerosis (SSc), WO 2011147810A1
2)Claudia Hirth-Dietrich, Peter Sandner, Johannes-Peter Stasch, Andreas Knorr, Degenfeld Georges Von, Michael Hahn, Markus Follmann, The use of sGC stimulators, sGC activators, alone and combinations with PDE5 inhibitors for the treatment of systemic sclerosis (SSc), WO 2011147810A1

Jin Li, Xiaoyu Yang, Jingwei ZHU, Minmin Yang, Xihan Wu, Method for synthesizing 1-(2-fluorobenzyl)-1H -pyrazolo[3,4-b]pyridin -3-formamidine hydrochloride, WO2013086935 A1

veerareddy Arava, Surendrareddy Gogireddy, An expeditious synthesis of riociguat, A pulmonary hypertension drug, Der Pharma Chemica, 2013, 5(4):232-239
cut paste from my earlier post
RIOCIQUAT
CAS NO 625115-55-1
Methyl N-[4,6-Diamino-2-[1-[(2-fluorophenyl)methyl]-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-pyrimidinyl]-N-methyl-carbaminate
9 APRIL2013
Bayer has been boosted by the news that regulators in the USA are fast-tracking the German group’s investigational pulmonary arterial hypertension riociguat.
The US Food and Drug Administration has granted priority review to the New Drug Application for riociguat, which Bayer filed in February on both sides of the Atlantic for PAH and a related condition, inoperable chronic thromboembolic pulmonary hypertension (CTEPH). The FDA bestows a priority review on medicines that offer major advances in care or that provide a treatment where no adequate therapy exists. The agency aims to complete its assessment within eight months from the submission of the NDA, rather than the standard 12 months.
Riociguat (BAY 63-2521) is a novel drug that is currently in clinical development by Bayer. It is a stimulator of soluble guanylate cyclase (sGC). At the moment Phase III clinical trialsinvestigate the use of riociguat as a new approach to treat two forms of pulmonary hypertension (PH): chronic thromboembolic pulmonary hypertension (CTEPH) andpulmonary arterial hypertension (PAH). Riociguat constitutes the first drug of a novel class of sGC stimulators
The submissions are based on two Phase III studies and riociguat, the first member of a novel class of compounds called stimulators of soluble guanylate cyclase (sGC), met its primary endpoint in both trials, a change in exercise capacity after 12- or 16 weeks respectively. The drug was generally well tolerated, with a good safety profile.
Kemal Malik, head of global development at Bayer Healthcare, noted that “to date, no approved pharmacological therapy exists for CTEPH and as a result there is an urgent unmet medical need for patients who are unable to undergo surgery. We hope that we will soon be in a position to provide patients and doctors with a new treatment option”.

If approved, riociguat would be going up against Actelion’s Tracleer (bosentan) and Gilead Sciences/GlaxoSmithKline’s Letairis/Volibris (ambrisentan). Actelion, which has dominated the PAH market, has already filed its follow-up to Tracleer, Opsumit (macitentan).

No comments:
Post a Comment