Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, helping millions with websites, 9 million hits on google, 2.5 lakh connections worldwide, P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
Thursday, 27 February 2014
Wednesday, 26 February 2014
Bhasma : The ancient Indian nanomedicine
Bhasma : The ancient Indian nanomedicine

Bhasma : The ancient Indian nanomedicine
Dilipkumar pal
Department of Pharmaceutical Sciences, Guru Ghasidash Vishwavidyalya (A Central University), Koni, Bilaspur – 495 009, Chhattisgarh
India
Ayurveda is the science made up of Veda (knowledge) and Ayush (life) i.e. knowledge of life. An Ayurvedic system adopts a holistic approach towards health care by balancing the physical, mental and spiritual functions of the human body. Rasa-Shastra (vedic-chemistry) is one of the parts of Ayurveda, which deals with herbo-mineral/metals/non-metals preparations called Bhasmas. Rasayana (immunomodulation and anti-aging quality) and yogavahi (ability to target drugs to the site) are characteristics of a properly made herbo-mineral/metals/non-metals preparation, which is also nontoxic, gently absorbable, adaptable and digestible in the body
Pal D, Sahu CK, Haldar A. Bhasma : The ancient Indian nanomedicine. J Adv Pharm Technol Res [serial online] 2014 [cited 2014 Feb 26];5:4-12. Available from: http://www.japtr.org/text.asp?2014/5/1/4/126980
DOI: 10.4103/2231-4040.126980
more info
Use of both bhasma (Residue after incineration – calcined preparation) as well as in pishti (powdered gem or metal) form along with appropriate herbs for treatment of critical ailments is a medicinal preparation in Ayurveda and to some extent Unani (both Indian branches of medical science using natural curative methods. The procedures for preparing these medicines are time-consuming and complicated.
Bhasma is a calcined preparation in which the gem or metal is converted into ash. Gems or metals are purified to remove impurities and treated by triturating and macerating in herbal extracts. The dough so obtained is calcinated to obtain the ashes.[2]^
Bhasma or vibhooti is the sacred ash from the dhuni or fire of a yogi or avadhoota, or from the sacrificial fire or yajna, where special wood, ghee, herbs, grains and other auspicious and purifying items are offered in worship along with mantras. It is believed that bhasma destroys sins (paap), and that it links us with the divine. It is called ‘bhasma’ because it has the power to consume all evils. Any matter, broken up through the process of fire is reduced to its ‘bhasmic’ form, which is infinitely more refined and pure than the original matter, devoid as it is of all impurities niranjan. The grossness of matter obscures the subtle essence inherent within it, just as wood hides fire and milk conceals butter and cheese, but when it is burnt (or churned in the case of milk) only the pure essence remains. Similarly, the great heat of tapasya and the churning of the mind in meditation reveals the underlying subtle spirit or atman.
Vibhuti
In certain circumstances Bhasma, ‘Vibhuti‘ (Sanskrit) and ‘Thiruneeru’ (Tamil) are synonymous.
Bhasmikaran
Bhasmikaran is a process by which a substance which is otherwise bioincompatible is made biocompatible by certain samskaras or processes (Puranik and Dhamankar, 1964e). The objectives of samskara are :- a) elimination of harmful matters from the drug b) modification of undesirable physical properties of the drug c)conversion of some of the characteristics of the drug d) enhancement of the therapeutic action(Puranik and Dhamankar, 1964e). Various steps involved in the preparation of bhasma(or bhasmikaran) are:- 1) Shodhan -Purification, 2) Maran – Powdering, 3) Chalan- Stirring, 4) Dhavan – Washing, 5) Galan- Filtering, 6) Putan- Heating, 7) Mardan- Triturating, 8) Bhavan- Coating with herbal extract, 9) Amrutikaran – Detoxification and 10) Sandharan- Preservation (Puranik and Dhamankar, 1964e). Selection of these steps depends on the specific metal. Sometimes there is an overlapping of the steps e.g. maran is achieved by puttan. Since the present thesis work is on bhasma, Bhasmikaran process is elaborated in details in the following paragraphs.
Steps of bhasmikaran
1. Shodhan: The principle objective of shodhan is to remove unwanted part from the raw material and separate out impurities( Vaiday and Dole 1996b). Metals obtained from ores may contain several impurities, which are removed by subjecting them to Shodhan process. In context of bhasma, shodhan means purifying and making the product suitable for the next step i.e. Maran. Ayurveda classifies shodhan into a) General process and b) Specific process.
General process for shodhan:
- “The sheets of metals are heated till red hot and are successively dipped into liquids like oil, buttermilk, cow’s urine etc. The procedure is repeated seven times”.
b. Specific process for shodhan For some metals a specific process is described for shodhan e.g. for purification of Jasad, the molten mass is poured in cow’s milk 21 times (Shastri K,1979b).
2. Maran : Maran literally means killing. As the name suggests in maran process, a change is brought about in the chemical form or state of the metal. This makes it to lose its metallic characteristics and physical nature. In short, after maran, metal can be converted into powder or other form suitable for administration. To convert various metals into a form appropriate for human consumption, several techniques have been employed which ultimately gave birth to concept: “Bhasma prepared by using Rasa i.e. mercury is the best, whereas the one prepared using herbs are of better quality and those prepared using Gandhak (sulfur) are of inferior quality. Thus there are 3 methods given for maran. It is carried out by heating the metal in presence of 1) mercury 2) plants and 3 ) sulfur.
When various maran procedures for different metals were reviewed, it was found that mercury is mainly used. The unique property of mercury to amalgamate with many metals must have been the reason behind its maximum use in the process of Bhasmikaran. Ancient practitioners might have found it as the most suitable chemical and therefore probably have mentioned that bhasmas using mercury are superior. Plants used in maran process may be serving as catalyst in the process or the minerals in the plants may be forming complexes with the metals. However, no such explanation can be obtained for the use of sulfur.
3. Chalan: Process of stirring during heating the metal is chalan. Stirring is carried out either with iron rod or stick made from a specific plant. As we know today, iron serves as catalyst in many chemical reactions. The phytoconstituents of plant stick may be enhancing the therapeutic effect. For example, stick of Neem is used for chalan process of Jasad bhasma, which is used topically for ophthalmic diseases. We can interpret the significance of this process now. Neem is an antiseptic (Puranik and Dhamankar, 1964h). Zinc is antiseptic, astringent and has ulcer healing property (Block et al., 1982b). These effects of both the constituents may impart the final product better therapeutic activity.
4. Dhavan: In this process, several water washes are given to the product obtained in the previous stage. Perhaps this is to remove the excess amounts of agents used in shodhan or maran stage. Such agents may adversely affect the quality of final product. Hence intermediates are washed with water, thereby water soluble constituents are removed (Puranik and Dhamankar, 1964h).
5. Galan: The product is then sifted either through a fine cloth or through sieves of suitable mesh so as to separate residual material larger in size (Puranik and Dhamankar, 1964h).
6. Puttan: The term puttan means ignition. The general term used for heating in the process of Bhasmikaran is Puta. A special earthen pot, Sharav is generally used for the process. It has two parts, each having a shape of soccer. Sharav is used for direct heating of the material. Its shallowness is useful in heating the material faster and uniformly. After keeping the material on the shallow surface, other part is used as a lid, by placing it in an inverted position. This Puttan process can be looked upon as the key step in manufacturing of bhasma. The classification of putta is primarily done on the basic nature of the process and is as under :- (Puranik and Dhamankar, 1964f) 1)Chandraputta 2) Dhanyarashiputta 3) Suryaputta 4)Bhugarbhaputta 5) Agniptuta.
Toxicity
Modern medical science finds that mercury is inherently toxic, and that its toxicity is not due to the presence of impurities. While mercury does have anti-microbial properties, and formerly waswidely used in Western medicine, its toxicity does not warrant the risk of using it as a health product in most circumstances.[3][4] The Centers for Disease Control and Prevention have also reported a number of cases of lead and mercury poisoning associated with rasa shastra containing Ayurvedic medicines.[5]
Literal and symbolic meaning of bhasma
The Sanskrit word bhasma literally means ‘disintegration’. Bha implies bhartsanam (to destroy), while sma implies smaranam (to remember). Bhasma is thus a reminder to us of the ephemeral nature of life. Also, if we wish to unite with God (or the ‘supreme self’) and remember him constantly, our ego or ‘little self’ has first to be disintegrated or burnt to ashes. Bhasma is a symbol of this process. It is also called raksha because it protects one from all fears. When applied to the forehead before sleep, it is said to keep away spirits or ghosts, whether external or those which manifest from the depths of the mind in the form of nightmares.
Bhasma symbolises the burning of our false identification with the mortal body, and freedom from the limitations of the painfully illusive cycle of birth and death. It also reminds us of the perishable quality of the body, which will one day be reduced to mere ashes. As it says in the Bible, “Ashes to ashes; soul to soul” – the body will return to dust but the soul will continue its journey until it unites with God. All the saints and sages beseech us to remember the ephemeral nature of our earthly existence. In the Rubayyat of Omar Khyyam the poet tells us to, “ . . . make the most of what we yet may spend, before we too into the dust descend, dust into dust, and under dust to lie.” Here he calls for us to seek the eternal, not the temporal. Ash or dust, on the other hand, can be said to represent permanency (or the soul itself), because the ash, just like imperishable truth, does not itself decay. The realised soul is said to rise from the ashes (of the individual self) as the mythical phoenix. The Sufis say, “To reach the goal we have to be burned with the fire of love, so that nothing remains but ashes, and from the ashes will resurrect the new being. Only then can there be real creation!”
The power of bhasma
Bhasma is also called ‘vibhooti’, because it gives spiritual power. The Sanskrit word, vibhooti means ‘glory’, as it gives glory to one who applies it, protection (raksha) from ill health and negative forces, and attracts the higher forces of nature. Another meaning of vibhooti is ‘healing power’, and it is widely used as a medicinal treatment in both Ayurveda and Chinese and Tibetan medicine, which are all ancient and profound systems for the rejuvenation of life. Gold, silver, copper, pearls, mica and other precious stones and metals have curative properties which can quite safely and most effectively be taken into the body after being reduced to ash using great heat.
In Indian villages you will find tantric healers called ojhas who say certain mantras over the ash, which the sick person then applies to the body or eats. These healers can take some earth in their hands, hold it up to the sun, repeat some mantras, and the earth turns into the most beautifully scented ash for curative purposes. Vibhooti is also the name given to siddhis (perfections or psychic powers), as it acts as a vehicle for them. Patanjali’s Yoga Sutras devotes an entire chapter to yogic siddhis. Vibhooti also means ‘dominion’, and is the subtle power lying behind creation, from which all things manifest. From vibhooti or bhasma, anything can be created by a tantric and aghora, because the potential of creation lies within it, and he has penetrated the law and controlled the elements.
Maha Yogi Shiva, father of tantra, is usually depicted naked in sadhana, his whole body covered in bhasma. The first verse of the Shiva Panchakshara Stotram gives the following description: Naagendrahaaraaya trilochanaaya, bhasmaangaraagaaya maheshwaraaya. Nityaaya shuddhaaya digambaraaya – ‘Salutations to the mighty three-eyed Shiva, eternal and pure, wearing the king of snakes as his garland, naked and besmeared with sacred ash.’ Some other names given to Lord Shiva are Bhasmashayaaya (abode of bhasma) and Bhasmabhootaaya (covered with bhasma). Covering the body with ash is considered to be an auspicious act for discovering one’s Shiva nature. Shiva is said to be responsible for mahapralaya, the dissolution of the universe at the end of each kalpa. At this time he dances his tandava nritya, the dance of destruction.
The great tantric siddha Avadhoota Dattatreya was referred to as Bhasma Nishta – one who loves bhasma. Bhasma is generally applied on the forehead, while many sadhus also apply it on the arms, chest and stomach. Some ascetics, especially nagas (naked ascetics) rub it all over the body. While applying it, many devotees also consume a pinch. Shaivites use only bhasma from cremated bodies, which is believed to be very powerful. Bhasma has the power of fire. Agni, the inner fire, scorches and reduces all impurities in the body. It is said that one who smears ash on the body is purified as if bathed in fire. This is known as ‘the bath of fire’. After smearing the body with ash, one should reflect on and realise the highest truth.
Tripundra
Sannyasins wear three lines of bhasma on the forehead. These three lines (tripundra), with a red dot of kumkum underneath, between the eyebrows, symbolise Shiva-Shakti (the unity of energy and matter that creates the entire seen and unseen universe). The lower line represents tamoguna (the state of inertia and darkness), the middle line represents rajas (activity and dynamism) and the top line represents sattwa (balance and illumination). The red dot or tika represents the power of shakti through sadhana, which can take the sadhaka beyond the three gunas or qualities to the state of turiya, the fourth dimension of existence. This is the state of trigunatita – beyond the three gunas.
Swami Niranjanananda says, “The three stripes represent the tradition of the paramahamsas. Jignasus are one stripe sannyasins, representing the drive and motivation to overcome the tamasic tendency. Karma sannyasins are given two stripes, representing their drive to overcome the rajasic along with the tamasic tendencies. Poorna sannyasins are given three stripes, which represent their motivation to transcend the three gunas and attain inner sublimation. The red dot represents the spiritual power or energy that gives us the strength to control the three gunas. It is the awakening of that shakti which is the real aim of sannyasa.”
Bhasma and tattwa shuddhi
Consciousness manifests as energy, which then condenses into matter. In the tantric practice of tattwa shuddhi, in order to experience consciousness free from matter, we reverse the process of evolution back through more and more subtle dimensions to its original cause. Bhasma is an integral part of tattwa shuddhi sadhana, as a symbol of purification on the physical, subtle and causal realms of consciousness. The process of disintegration undergone in tattwa shuddhi is the breaking down of conscious awareness. Just as we reduce matter to its bhasmic form, the ‘fire’ of this practice leads us to the realisation of our essential essence. The stages of pratyahara (sense withdrawal) and dharana (concentration) take us through the more subtle states of consciousness, culminating in samadhi, the ultimate experience or ‘Shiva consciousness’. The journey is from gross matter to pure consciousness.
At the end of the practice of tattwa shuddhi, bhasma is applied to the forehead with the repetition of mantras. It is taken on the middle and ring fingers and wiped slowly on the forehead from left to right, repeating the mantra Om Hraum Namah Shivaya. Sannyasins use the index, middle and ring fingers, and repeat the mantra Om Hamsa. The bhasma used in tattwa shuddhi is prepared from gobar or cow dung. The word gobar literally means ‘gift from the cow’; it is also known as go-maya. The cow is a pure and sacred animal, full of auspicious qualities. It is even said to contain all the devas and devatas within it. Not only does gobar have mystical qualities, but it also contains useful hormones with germicidal properties. The word go also means ‘senses’. So bhasma is also symbolic of the disintegration of the senses which keep us trapped and bound in the gross material world. The transformation of gobar to bhasma is parallel to the transformation from the material world to cosmic consciousness that we find in tattwa shuddhi.
Panchagni bhasma
During the Sat Chandi Mahayajna, and on other auspicious occasions at Rikhia Dham, devotees receive the precious prasadam of panchagni bhasma. This is much prized by sadhakas, because as it has the power of Swami Satyananda’s sadhana behind it, it quickly helps to raise the consciousness at the time of mantra japa and other sadhana when applied to the forehead. Just keeping it in the pooja room is auspicious. This bhasma given is from the Maha Kaal Chita Dhuni, where the previously fierce fires of Sri Swamiji’s panchagni tapasya now lie smouldering quietly under ashes in their shanta roopa or peaceful form. Dhuni is the yogi’s fire, which is the witness or sakshi to his sadhana. It is also where he cooks, takes warmth, and chants the name of God. (Maha means ‘great’, kaal is ‘time’ and chita is ‘consciousness’).
This akhanda dhuni, eternal fire, has been burning in Sri Swamiji’s pooja area ever since he first came to Rikhia in 1989 and devotees come daily for its darshan. It was the centre and support of his life during his austerity, and is the very heart of the Rikhia Ashram (next to Sri Swamiji himself). Although Sri Swamiji no longer goes to this area, the fire is still tended daily. The ashes are moved to the side and the burning embers taken out. Balls of dried cow dung mixed with purifying herbs (vanaspati) are then placed inside along with fresh wood. The embers are then replaced, and the whole area is covered over once more with the ashes. From time to time the ash is removed, carefully sieved through fine cloth, and given as prasadam (that which has been blessed by a divine power.
For the panchagni sadhana itself, Sri Swamiji prepared his own bhasma to protect his body from the great heat, according to the formula prescribed in the Devi Bhagavat Purana. This special bhasma is called mahabhasma and is made from pure cow dung cakes, reeds and ghee. It is treated eleven times with many herbs, honey and other ingredients, being re-burnt each time. Bhasma is smeared on the body only during the first few days of the panchagni sadhana, and is applied in the morning. Sri Swamiji’s dog-cum-companion Bholenath, in whom he manifested the spirit of Bhairava, also took part in the tapasya. “Alsatian dogs can’t bear the heat,” commented Sri Swamiji. “I would put bhasma on him in the morning and he would sit with me.”
Tantric siddhas like Maha Yogi Shiva, Avadhoota Dattatreya and Sri Swamiji are extremely rare beings, and a gift to us beyond our understanding. They belong to a great tradition and leave behind for us a great spiritual legacy. The parampara, the line of avadhootas (those who have become immortal), continues, just as the Mahakaal Chita Dhuni continues to smoulder, unseen beneath the symbol of their glory, their bhasma – the sacred ash.
Abhrak Bhasma
Strengthens body, ligaments & Saptadhatu, effective in raktapitta, vat diseases and joint pain. Relieves problems related to chronic hyperacidity like stomache, headache etc.
Details
The Indian ayurved philosophy is founded on three basic classifications of human body known as doshasand its well-being:
Vat - related to the central nervous system and gastric tract,
Pitta -digestion and metabolism, governs movement of heat in the body and
Kapha - concerned with structure, stability and fluid balance in the body.
Indian herbal medicines are designed for the specific maintenance of these respective doshas or the aspects of the human body.
Abhrak Bhasma or Sahastraputi is an ancient Indian ayurvedic medicine which is trusted worldwide for healing Vat related issues of the human body. It is also found extremely effective for treating heart related issues.
Abhrak is known to have a high penetrative property which spreads in the body at a faster rate and impacts micro tissues quickly.That is why this herbal medicine is supremely effective in cell regeneration. When used as an alterative, it strengthens ligaments. It is known for rapidly increasing the production of T-cell phagocytes, antibacterial components, which are responsible for a strong immune system.
Kesar Herbals has been in the ayurvedic medicine manufacturing business for about 25 years. This company is one of the foremost and trusted ayurvedic medicine suppliers in India.The concept was let entire mankind benefit from the ancient herbal wealth of India.
Composition:
Purified Mica, Magnesium, Iron, Potassium, Calcium and Aluminium.
Major Benefits of Abhrak Bhasma
- It finds its application in curing diseases like Hepatitis, Tuberculosis, Asthma and Plague.
- Abhrak Bhasma is also recommended for breathlessness due to chronic Bronchitis, Asthma, and even Chest Congestion. When taken in the initial stage of TB, it can completely cure it.
- It is beneficial to all the seven dhatus (energy elements) in the body.
- It rejuvenates the human mind.
- It improves blood circulation, improves the general tone of tissues and conductivity.
- It cures erectile dysfunction and impotency in men.
- Abhrak is hematinic, which means increases the count of red blood cells. This also increases oxygen carrying ability of the cells.
- It helps in curing jaundice and anemia.
- It restores damaged tissues and maintains their health.
- It is highly effective in cases of bone marrow depletion and hepatic dysfunction.
- It is effective in curing respiratory tract infections.
- It is beneficial for curing Bells Palsy and dysentery.
- It is beneficial in curing anemia, pernicious and azoospermia.
- Also, helps in controlling many types of veneral diseases like EBV, HIV, Lupus bronchitis and pneumonia.
- It cures breast cancer.
- It is also known to cure chronic hyperacidity like stomach-ache and vomiting with blood.
Suvarna Bhasma
Boosts immunity, Acts as a nervine tonic , Indicated in old age debility, urinal disorders, anemia, Shwas, Helps to improve complexion, Kas & chronic fever etc.
Details
Swarna Bhasma is an ancient Indian ayurvedic medicine that enjoys wide popularity and application.Gold has been accepted as most useful ingredient in Indian ayurvedic medicine system. SwarnaBhasma is used for the effective treatment of Gonorrhoea and Syphilis.
Fortified with potent ayurvedic herbs it is effective for maintaining a healthy life-style and relieves stress.Bhasma denotes the metal based medicine prepared from metals after scientific process to harness the hidden richness of raw metals for therapeutic effects. Swarna Bhasmaor Gold Ash is a therapeutic form of gold metal of nano-sized particles that is one of the most treasured ayurvedic medicines in the world.
It has multi-purpose ayurvedic medicine: it can be used as an antacid, haematinic, and alterative. It is highly effective as a cardiac tonic, maintaining the vitality of the human heart. It is a general vitality booster.
Kesar Herbals firmly believes in the power of nature in healing various ailments. Ayurvedic medicines from India are a gift from nature itself, without any side-effects, and hence they are trusted world-wide.Through scientific processes, nature’s best healing powers from herbs are obtained and used for medicinal purposes, helping million people worldwide. Kesar Herbals is involved in consulting, manufacturing and supplying FDA approved ayurvedic medicines for over three decades.
Major Benefits of Swarna Bhasma
- Swarna Bhasma improves resistance power in human body.
- It is responsible for boosting immunity and strengthening the human body.
- It can be used on a regular basis as an alterative and acts as a nerve tonic.
- Swarna Bhasma is excellent in treating Anemia, and chronic fever.
- Due to its gold base, it is helpful in improving complexion.
- Swarna Bhasma eradicates all chronic disorders, when used consistently.
- It is indicated in chronic fever, cough, Asthma, urinary disorders, sleeplessness and weak digestion.
- It strengthens weak muscles, debilities associated with old-age.
- It is excellent for treating Tuberculosis.
- It increases sexual power.
- Swarna Bhasma slows down the process of degeneration of tissues thus helps to prevent premature ageing.
- It helps prevent symptoms of ageing like wrinkles, dullness of skin, dark circles around the eyes, debility, fatigue, etc.
- Swarna Bhasma strengthens tissues and ligaments of the human body.
- It is highly effective in maintaining vigor, vitality and stamina.
Hirak Bhasma
effective against immunity disorders, Bone marrow depression and arthritis, increases stamina, vitality and strength
Ayurvedic medicinal science has been at the forefront of solving most diseases and disorders of the human body because of the purity of its ingredients- this forms the core of the ancient Indian healing system. Ayurvedic medicines and herbal products are effective because of the composition or the formulation of herbs. One such effective ayurvedic medicine is the Hirak Bhasma, which contains diamond ash.
According to Ayurveda, diamond ash is very useful in cancers, immunity disorders, Crippling Rheumatoid arthritis, and even bone marrow depression. Hirak Bhasma is known for toning heart muscles and avoiding cardiovascular syndromes and diseases. It is highly effective as a tonic and alterative, which means, it can be used regularly to strengthen immunity, aid stamina and overall vitality or the functionality of the human body.
Hirak Bhasma is made with diamond- the most precious as well as the hardest gemstone known to mankind. In its healing formulation, diamond is known since ages to make the human body stronger. It is also known for sharpening the human mind by strengthening memory power.
The effectiveness of ayurvedic medicines depends on the company. So be careful in selecting the brand for these medicines and products. Kesar Herbals has been in the ayurvedic consulting space and even manufacturing and supplying of ayurvedic medicines and herbal products for over 25 years. The trust and good will it represents in the field of Ayurveda is affirmed by its customers the world over. It is important to ascertain the brand equity of the company before purchasing these medicines.
Composition:
Hirak Bhasma (diamond ash), Kumari Rasa (Aloe Vera Juice), Gulab Jala (Rose Water), Sunthi Kwath (Dry Ginger Dicoction)
Benefits:
- Hirak Bhasma is known the world over to make the human body stronger and the mind sharper.
- It is also recommended for the well-being of the human heart.
- It helps in toning the muscles of the heart.
- Its regular consumption as a tonic or an alterative, under medical prescription or supervision is known for toning the heart muscles.
- Hirak Bhasma also increases stamina, vitality and strength.
- It is highly effective against immunity disorders.
- It is known for curing Crippling Rheumatoid
- It is effective against Bone marrow depression and arthritis.
- Hirak Bhasma is also known to cure one of the most dangerous and lethal diseases known to mankind: cancer. When used in the initial stages, under medical supervision, it can cure cancerous cells.
- Ayurvedic formulary of India , 1978a
- Major Herbs of Ayurveda, Elizabeth M. Williamson. ISBN 0-443-07203-5
- http://www.atsdr.cdc.gov/toxprofiles/tp46.html
- http://toxnet.nlm.nih.gov/cgi-bin/sis/search/f?./temp/~jcaJ1Z:2
- CDC Morbidity and Mortality Weekly Report: Lead Poisoning Associated with Ayurvedic Medications — Five States, 2000–2003
Tuesday, 25 February 2014
BIOSIMILARS REGULATORY ASPECTS

Biological medicines are already becoming an increasingly important part of health care. With patent expiries on originator biological products, biosimilars are also increasingly become a part of this future. In fact, by 2020 twelve of the top-selling biologicals will have lost patent protection, opening up an estimated US$24 billion in EU sales and US$30 billion in US sales.
Biologicals have potential to reach up to 50% share in global pharmaceutical market in the next few years.
India is one of the leading contributors in the world biosimilar market and is the third-largest in the Asia-Pacific region, after Australia and China. India has demonstrated high acceptance of biosimilars, which is reflected in the 40 biologicals marketed in India, of which 25 are biosimilars The Indian biotechnology industry is also gaining momentum, with revenues of over US$4 billion in 2011, and which are projected to reach up to US$580 million by 2012.
While small molecule drugs are ideal for generics replication, biological drugs are not so simple. Biological drugs are usually large, complex molecular structures derived from or produced through a living organism, making them very difficult to replicate
Currently there is considerable interest in the legislative debate around generic biological drugs or “biosimilars” in the EU and US due to the large, lucrative market that it offers to the industry. While some countries have issued a few regulatory guidelines as well as product specific requirements, there is no general consensus as to a single, simple mechanism similar to the bioequivalence determination that leads to approval of generic small molecules all over the world. The inherent complex nature of the molecules, along with complicated manufacturing and analytical techniques to characterize them make it difficult to rely on a single human pharmacokinetic study for assurance of safety and efficacy.
In general, the concept of comparability has been used for evaluation of the currently approved “similar” biological where a step by step assessment on the quality, preclinical and clinical aspects is made. In India, the focus is primarily on the availability and affordability of life-saving drugs. In this context every product needs to be evaluated on its own merit irrespective of the innovator brand. The formation of the National Biotechnology Regulatory Authority may provide a step in the right direction for regulation of these complex molecules. However, in order to have an efficient machinery for initial approval and ongoing oversight with a country-specific focus, cooperation with international authorities for granting approvals and continuous risk-benefit review is essential. Several steps are still needed for India to be perceived as a country that leads the world in providing quality biological products.

We are now in the twenty-fifth anniversary year of the Drug Price Competition and Patent Term Restoration Act of 1984 (better known as the Hatch Waxman Act), the landmark US regulation that jump-started the generic pharmaceutical industry. The legislation provided the required impetus to make not just cheaper price alternative medicines available to US consumers but stimulated the emergence of the Indian pharmaceutical industry which is now the dominant supplier of generic drugs to the USA.
The regulatory pathway for bringing generic drugs to market is the abbreviated new drug application (ANDA) process which relies on proving bioequivalence to the listed reference product and showing equivalent product quality. Since duplication of proof of safety and efficacy in the preclinical and clinical setting is not required, there are significant cost savings in bringing a copy of a small chemical molecule to market. This model has been so successful in economic terms that almost 7 out of 10 prescriptions in the US are now generic and for the vast majority of products there is no concern in substitution of a generic equivalent for a brand-name prescription.1
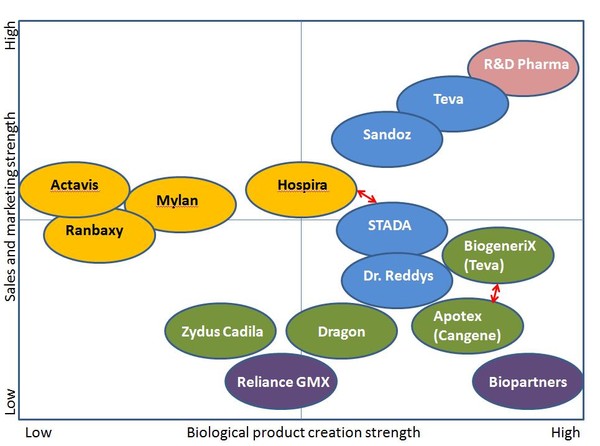
The success story of generic small molecule drugs has stimulated interest in the pharmaceutical and biotech industry for applying an analogous approach towards the highly lucrative biologics business. But biologic drugs are very different from small molecules both in their final form and in the process required to produce and control their quality. It is therefore difficult to find a simple, precise “regulatory” definition of biologics. However, biologics are generally understood to be drugs derived from an organic source. Thus, biologics may be obtained or created from living organisms, either naturally or via genetic manipulation or are manufactured from building blocks of living organisms. They demonstrate considerable molecular complexity and may comprise a diversity of molecular forms. Their larger size and heterogeneity make it difficult for complete characterization via physicochemical analysis which is possible for synthetic chemical entities. In general, biologic drugs are more expensive and the cost of a yearly treatment may run into thousands of dollars for some. They are therefore ideal targets for developing cheaper alternatives.
US FDA definition - A “biological product” means a virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, allergenic product, or analogous product, or arsphenamine or derivative of arsphenamine (or any other trivalent organic arsenic compound), applicable to the prevention, treatment, or cure of a disease or condition of human beings (Public Health Service Act Sec. 351(i)).
Given the complexity of the final biologic product, it is clear that the nature of the manufacturing process is also complicated. In addition to aspects that are disclosed in regulatory applications, there may still be several aspects which might be held as trade secrets, thereby making it practically impossible for another company to make an identical copy of a biologic drug. While changing a host cell line or vector will definitely impact the product, effects of minor changes like temperature used in the manufacturing process may have an effect on the final characteristics of the biologic drug, including its safety and efficacy. It has been stated often that for a biologic, “the process defines the product”. Thus, while it may be possible to make a similar product, it may not be truly bioequivalent. As a result, even the term used to describe these similar biologic drugs has not been standardized globally. While the parallel term for a biologic generic may intuitively be “biogeneric”, the accepted term in Europe and Canada is “biosimilar” and the preferred term in the US is “follow-on biologic”.
Given these differences among innovator biologics and their “similar” counterparts, there is considerable hesitation on the part of the regulatory agencies to follow an abbreviated approval path similar to one widely used for generic small molecules.
Status of biosimilar regulation in Europe
EMEA Guidelines for Similar Biological Medical Products ( CHMP/437/04, 30 October 2005).2
EMEA's Guideline on Similar Biological Medicinal Products Containing Biotechnology-derived Proteins as Active Substance: Nonclinical and Clinical Issues (EMEA/ CHMP/BMWP/42832/2005).3
EMEA's Guideline on Similar Biological Medicinal Products Containing Biotechnology-derived Proteins as Active Substance: Quality Issues, EMEA/CHMP/49348/05.4
In Europe, the Committee for Medicinal Products for Human Use (CHMP), the European Medicines Agency (EMEA) led the way for biosimilars, by issuing its first specific regulatory guidance in October 2005. Two general guidance documents addressing quality and nonclinical and clinical perspectives (June 2006), five product-specific annexes on nonclinical and clinical issues (June-July 2006) and a manufacturing change comparability guideline (November 2007) are now available.
Biosimilars
Testing the bioequivalence of biosimilars differs from that of standard generics. Bioequivalence testing procedures for biosimilars are to be performed against the originator product as a control (reference) and include preclinical and clinical testing [2].
Testing the bioequivalence of biosimilars differs from that of standard generics. Bioequivalence testing procedures for biosimilars are to be performed against the originator product as a control (reference) and include preclinical and clinical testing [2].
In the Biologics Price Competition and Innovation (BPCI) Act, a biosimilar product is defined as a product that is ‘highly similar’ to the reference product, notwithstanding minor differences in clinically inactive components and there are no clinically meaningful differences in terms of safety, purity and potency. However, little or no discussion regarding how similar is considered ‘highly similar’ is given in the BPCI Act.
For biosimilars, most of which have long half-lives, crossover study would be ineffective and unethical. This is due to the fact that a crossover study requires a wash-out period (which would be long for biosimilars with long half-lives) where the patient is not allowed to take the drug and therefore will have no treatment for their condition. On the other hand, parallel-group studies are required, but these studies do not provide an estimate of within-subject variation. For a parallel-group study, each drug is administered to a different group of subjects. Thus, we can only estimate total variance (between and within-subject variances), not individual variance components. This makes an evaluation of interchangeability difficult.
Statistical tests that may be used to asses biosimilarity are Shuirmann’s two one-sided tests procedure or the confidence interval approach.
Status of Biosimilar Regulation in US
In US, in March 2009, Representative Henry Waxman introduced H.R. 1427 to the Congress “Promoting Innovation and Access to Life-saving Medicines Act”, which authorizes FDA to approve follow-on biologics in an abbreviated manner. It has market exclusivity clauses with time frames similar to ones used currently for drugs. Other bills are expected to follow in the 2009 legislative agenda in order to establish a pathway for approval of these follow-on biologics. The contentious issues as expected, are focused around the duration of exclusivity benefits granted to innovators. The issue of substitutability of followon biologics for reimbursement is also an important one as the legislators debate the merits of each bill.
Korea and Singapore have released draft guidelines on biosimilars in 2009. The Singapore guideline is derived mainly from the EMEA guidelines and defines a similar biological/ biosimilar product as “a biological medicinal product referring to an existing registered product, submitted for medicinal product registration by an independent applicant, and is subject to all applicable data protection periods and/or intellectual property rights for the original product”. In addition to specifying the requirements for biosimilars, the guidance requires that the product have prior approval in countries such as Australia, Canada, EMEA or US.
Indian scenario
The Indian biotech industry is a thriving industry which got its start from vaccine manufacturing. In addition to meeting domestic demands, the Indian vaccine industry also fulfils export requirements to a large extent. Therefore it is evident that manufacturing expertise in producing biologic products of required export quality already exists in the country. What is not readily evident is whether these products can prove to be “comparable” to innovator products when we look into all categories of biologics.
The evolution of regulations governing pharmaceuticals in India has historically been driven by the need to make essential medicines accessible to patients. Access encompasses availability and affordability. It applies to medicines for all indications, acute and chronic illness, small molecules and biologics alike. The absence of product patent regulations for drugs marked a period in the country's history where it was imperative to make inexpensive medicinal products available to the masses – it did not matter whether these products were innovator-made or copies thereof. In the post-TRIPS era however, there is need to offer and enforce adequate protections for patentable drugs, particularly biologics that inherently involve huge investments in R & D, manufacture and clinical development.

Today, several biologics have been approved in India , including recombinant human insulin, recombinant human erythropoietin (EPO), interferon (IFN), granulocyte colony stimulating factor (GCSF). The versions of biologics available in India are typically products whose patents have expired or do not exist in India. Therefore, from a technical standpoint, there is no concern about patent infringement regarding these (there are no patents in India for these products). If a biosimilar results in a price drop of 30%, it is a significant improvement to patients who may now be able to afford this generic version of a life-saving drug. In many ways, the debate about biosimilars that rages across the developed world and regulated markets is irrelevant to India where the central concern revolves around access.
Partly due to the dearth of appropriate resources and experience, Indian regulators have sought to mimic regulations already in use in the developed world without much customization. A host of agencies have been created to address the issues brought forth by biologics.
Basic facts about biosimilars.
Biotechnological drugs have become an essential part of modern pharmacotherapy and are expected to reach a 50% share in the pharmaceutical market in the next few years. The expiry of patent protection for many original biotechnological medicines has led to the development of what are called biosimilars or follow-on biologics. Biosimilars attempt to copy the original technology leading to the production of innovative biotechnological medicines to obtain a product which is similar to the original one. The first two biosimilars have recently been approved in the European Union and one application was rejected. Many more biosimilars will likely see approval in the near future. Our experience with biosimilars has been very limited to date and long-term safety data including immunogenicity are not available. Although biosimilars will likely lower the cost of modern therapies there are issues which have to be discussed at this stage among physicians regarding in particular the differences between biosimilars and generics of the classical chemical drugs, need for appropriate regulations as well as identification of potential problems with biosimilars. Other specific problems which will also be addressed in this review are safety of biosimilars, pharmacovigilance, automatic substitution, naming and labeling/prescription rules. 7
List of agencies
- Indian Council for Medical Research (ICMR)
- Central Drugs Standard Control Organisation (CDSCO)
- Department of Biotechnology (DBT)
- Genetic Engineering Approval Committee (GEAC)
- Recombinant DNA advisory Committee (RDAC)
- Review Committee on Genetic Manipulation (RCGM)
- Institutional Biosafety Committee (IBSC)
- National Centre for Biological Sciences
- National Control Laboratory for Biologicals
Notwithstanding the above, there is clarity on the fact that biologics and drugs need to be scrutinized differently. With this in mind, the DBT has been given the mandate to set up the National Biotechnology Regulatory Authority (NBRA). This is envisaged as an independent, autonomous and professionally led body to provide a single window mechanism for biosafety clearance of genetically modified products and processes.
Before such an organization can be effectively implemented, it will be necessary to put in place appropriate new legislation, namely the “National Biotechnology Regulatory Act” or the NBR Act. Draft establishment plan and “Draft National Biotechnology Regulatory Bill, 2008” are currently available on the DBT website for comments. The responsibility of consolidating the feedback has been entrusted to Biotech Consortium India Limited (BCIL). The draft bill envisions the scope of this authority to encompass research, manufacture, import and use of genetically engineered organisms and products derived thereof.
Biosimilars: how similar or dissimilar are they?
The imminent expiry of patents on biological medicinal products, such as epoetin alfa in 2006, has significant implications for nephrology in Australia. The purpose of this review is to examine the differences between biosimilars (similar biological medicinal products) and generic low molecular weight (chemical) drugs. The approach that regulatory agencies, including the European Medicines Agency (EMEA) and the Therapeutic Goods Administration (TGA), are taking towards biosimilars is also discussed. Biosimilars differ from generic chemical drugs in many important ways, including the size and complexity of the active substance, the nature of the starting materials (cell banks, tissues and other biological products), and the complexity of the manufacturing processes. Therefore, it has been acknowledged by the EMEA that established legal and regulatory principles of 'essential similarity' that are applied to standard chemical generics cannot be readily applied to biosimilars. One of the key areas of concern with the introduction of biosimilars into the field of nephrology will be guaranteeing the safety and efficacy of biosimilars. New manufacturers will need to ensure that their biopharmaceutical has a similar efficacy and safety profile to the innovator product through more extensive clinical trials than the limited testing done for generic versions of low molecular weight chemical medicines. 6
Safety
The primary importance of the manufacturing process was highlighted when a slight change in the production process of an originator recombinant erythropoietin resulted in patients developing pure red cell aplasia.
The primary importance of the manufacturing process was highlighted when a slight change in the production process of an originator recombinant erythropoietin resulted in patients developing pure red cell aplasia.
To try to address this possible safety issue, guidelines from EMA on comparability of biosimilars state that preclinical data must be insufficient to demonstrate the immunological safety of some biosimilars. This means that safety must be demonstrated in cohorts of patients enrolled in clinical trials and using post marketing surveillance.
The challenge of biosimilars.
The purpose of this report was to review issues associated with the introduction of alternative versions of biosimilars used in the oncology setting.Data were obtained by searches of MEDLINE, PubMed, references from relevant English-language articles, and guidelines from the European Medicines Agency.When biosimilars are approved in EU, they will be considered 'comparable' to the reference product, but this does not ensure therapeutic equivalence. Inherent differences between biosimilars may produce dissimilarities in clinical efficacy, safety, and immunogenicity. Switching biosimilars should be considered a change in clinical management. Regulatory guidelines have been established for some biosimilar categories but, because of the limited clinical experience with biosimilars at approval, pharmacovigilance programs will be important to establish clinical databases. Guidelines also provide a mechanism for the extrapolation of clinical indications (approved indications for which the biosimilar has not been studied). This may be of concern where differences in biological activity can result in adverse outcomes or when safety is paramount (e.g. stem cell mobilization in healthy donors). These issues should be addressed in biosimilar labeling.Biosimilars should provide cost savings and greater accessibility to biopharmaceuticals. A thorough knowledge surrounding biosimilars will ensure the appropriate use of biopharmaceuticals.
Pharmacovigilance
Due to the limited clinical database at the time of approval of a biosimilar, vigorous pharmacovigilance is required. EMA guidelines require pharmacovigilance programmes to monitor the safety of biosimilar products post-approval.Substitution
For small molecule generics the issue of substitution is easy, since they are considered identical to the originator molecule. This, however, is not the case for biosimilars, which are large complex molecules prone to heterogeneity.In the US, the BPCI Act gives FDA the authority to designate a biosimilar as interchangeable with its reference product. This means that the biosimilar may be substituted for the originator product by the pharmacist without reference to the prescribing physician. This is not the case, however, in the EU, where decisions on interchangeability are not made by EMA, but at a national level.
Global concerns regarding product safety and quality
Every drug/biologic manufacturer needs to own the responsibility for putting a high quality, safe drug on the market, after appropriate review and approval by the concerned regulatory authority. While the safety of original biologics products is assured by the innovator by adherence to rigorous standards required for approval, the resistance towards biosimilars on the part of regulators, stems from the concern that an abbreviated approval process may not be adequate to ensure safe performance of the product in the market. For a manufacturer looking to get into the biosimilar market, he needs to overcome major challenges in making a complex product, getting regulatory approval by satisfying stringent criteria and then selling it in the market. Typically, facilities required for manufacture of biologicals are very expensive and the kind of infrastructure required to meet high regulatory expectations is limited to only a few companies. Clinical trial expenditure and ongoing analysis requires compliance to pharmacopoeial monographs when available and access to reference standards, which are not always available.
The cornerstone of generic drug approvals has been the concept of bioequivalence, using equivalence of pharmacokinetic parameters as surrogates for clinical efficacy. But in the context of biosimilars, the concept of comparability is the one used to make such an evaluation. Comparability protocols are used for chemistry, manufacturing and controls (CMC) sections to make the case on the quality aspects of the product. Preclinical testing requires knowledge of study designs used by innovator in order to truly compare performance of the biosimilar. For clinical evaluation, at least one clinical comparability trial is required to demonstrate comparability (non-inferiority in terms of efficacy to innovator and comparable safety profile). But long term safety issues remain unaddressed for biosimilars, requiring thorough postmarketing studies and pharmacovigilance and adequate risk management plans.
In terms of preclinical studies, for biologics, pharmacodynamic endpoints are more relevant than pharmacokinetics, which is the key measure with small molecules. For animal safety studies, choice of appropriate animal species and duration of studies are important criteria for proving comparability. Clinically, comparative PK/PD study is required to compare the reference and biosimilar product. However, clinical trial design selection and a thorough understanding and a priori statement of margins chosen for comparability must be stated for meaningful evaluation of data.
Follow-on biologics: challenges of the "next generation".
The imminent patent expiration of many biopharmaceutical products will produce the possibility for generic versions of these therapeutic agents (i.e. biosimilars). However, there are a number of issues that will make approval of biosimilars much more complicated than the approval of generic equivalents of conventional pharmaceuticals. These issues centre on the intrinsic complexity of biopharmaceutical agents, which are recombinant proteins in most cases, and the heterogeneity of proteins produced by different manufacturing processes (i.e. differences in host cells, purification and processing, formulation and packaging). The increased occurrence of antibody (Ab)-mediated pure red cell aplasia (PRCA) associated with a change in the formulation of one particular epoetin-alpha product highlights the potential for increased immunogenicity of recombinant proteins with different formulations, or those manufactured by different processes. Thus, verification of the similarity to or substitutability of biosimilars with reference innovator biopharmaceutical products will require much more than a demonstration of pharmacokinetic similarity, which is sufficient for conventional, small molecule generic agents. Regulatory requirements for the approval of biosimilars have not yet been fully established, but preliminary guidelines from the European Agency for the Evaluation of Medicinal Products (EMEA) state that the complexity of the product, the types of changes in the manufacturing process, and differences in quality, safety and efficacy must be taken into account when evaluating biosimilars. For most products, results of clinical trials demonstrating safety and efficacy are likely to be required. In addition, because of the unpredictability of the onset and incidence of immunogenicity, extended post-marketing surveillance is also important and may be required. 10
Statistical assessment of biosimilar products.
Biological products or medicines are therapeutic agents that are produced using a living system or organism. Access to these life-saving biological products is limited because of their expensive costs. Patents on the early biological products will soon expire in the next few years. This allows other biopharmaceutical/biotech companies to manufacture the generic versions of the biological products, which are referred to as follow-on biological products by the U.S. Food and Drug Administration (FDA) or as biosimilar medicinal products by the European Medicine Agency (EMEA) of the European Union (EU). Competition of cost-effective follow-on biological products with equivalent efficacy and safety can cut down the costs and hence increase patients' access to the much-needed biological pharmaceuticals. Unlike for the conventional pharmaceuticals of small molecules, the complexity and heterogeneity of the molecular structure, complicated manufacturing process, different analytical methods, and possibility of severe immunogenicity reactions make evaluation of equivalence (similarity) between the biosimilar products and their corresponding innovator product a great challenge for both the scientific community and regulatory agencies. In this paper, we provide an overview of the current regulatory requirements for approval of biosimilar products. A review of current criteria for evaluation of bioequivalence for the traditional chemical generic products is provided. A detailed description of the differences between the biosimilar and chemical generic products is given with respect to size and structure, immunogenicity, product quality attributed, and manufacturing processes. In addition, statistical considerations including design criteria, fundamental biosimilar assumptions, and statistical methods are proposed. The possibility of using genomic data in evaluation of biosimilar products is also explored.15
A way forward for India
In today's scenario, India needs to focus on quality of each biological product per se, whether that is demonstrated through comparability or by its own merit; and assurance of safety through appropriate regulatory review and approval of available data.
Irrespective of the authority entrusted to oversight of biologics, the debate on appropriate level of regulatory scrutiny for biologics will continue to focus on requiring adequate characterization while balancing cost, with the overall goal of having a much needed product on the market with reasonable assurance of efficacy and safety. Intense discussion on publication of appropriate monographs in the Indian Pharmacopeia and availability of reference standards continues amidst regulatory circles. Indian manufacturers have always sought to enter new markets and have voluntarily raised the bar in order to secure approvals for their products in the regulated markets where profit margins are high. From a facility infrastructure and systems point of view, most companies eyeing the regulated markets for their products will most likely fulfil expectations. State-of-the art analytical techniques are available within the industry. Therefore from a quality standpoint, biologic products made in India should not have any trouble in meeting market expectations. However, physicochemical characterization of a biologic product and compliant facilities form only one part of the evaluation required to demonstrate product comparability.
The practical way forward for approval of biosimilar products in India would have to be unique to the Indian context while staying rooted to scientific basics and keeping in mind the needs and limitations of the country. The large majority of biosimilars introduced in India would be products whose patents have expired and where the “original innovator” product may not be approved in the country. It is also possible that no patent exists in India for some products and therefore , originator and similars coexist. For all products, the question of available reference standards and monographs would continue to remain. The next wave of biologics of commercial interest to the industry will become a burning issue where the regulator cannot expect to wait to see how the legislation is crafted in the US or elsewhere before making a move.
In my opinion, it seems that India, having the benefit of in-house (in-country) expertise in the area, should utilize the various agencies currently entrusted with splintered tasks and responsibilities to come up with working group or taskforce whose goal is to develop product-specific guidelines for approval. These can be developed using available worldwide regulatory knowledge by signing appropriate MOUs if necessary, studying the scientific literature and current industry standards and practice with respect to characterization, focusing on specific areas of unique concern for each product and proposing an approval path. These guidelines can be widely disseminated in the community. There will still be grey areas that need clarification and in such cases, a system for formal meeting with members of the working group/taskforce can be instituted, similar to the scientific advice that is currently available through the EMEA or individual European country competent authorities.
As a nation that takes pride in being the “exporter to the world” in the arena of pharmaceuticals, it behooves not just the regulators but all those in the regulatory affairs profession in India to support such initiatives to make life-saving products available to our countrymen that are unquestionably of the highest standards in terms of quality, safety and efficacy such that we become the supplier of choice when it comes to exporting biosimilars to markets in every corner of the world.
Biosimilar therapeutics-what do we need to consider?
Patents for the first generation of approved biopharmaceuticals have either expired or are about to expire. Thus the market is opening for generic versions, referred to as 'biosimilars' (European Union) or 'follow-on protein products' (United States). Healthcare professionals need to understand the critical issues surrounding the use of biosimilars to make informed treatment decisions.The complex high-molecular-weight three-dimensional structures of biopharmaceuticals, their heterogeneity and dependence on production in living cells makes them different from classical chemical drugs. Current analytical methods cannot characterize these complex molecules sufficiently to confirm structural equivalence with reference molecules. Verification of the similarity of biosimilars to innovator biopharmaceuticals remains a key challenge. Furthermore, a critical safety issue, the immunogenicity of biopharmaceuticals, has been highlighted in recent years, confirming a need for comprehensive immunogenicity testing prior to approval and extended post-marketing surveillance.Biosimilars present a new set of challenges for regulatory authorities when compared with conventional generics. While the demonstration of a pharmacokinetic similarity is sufficient for conventional, small-molecule generic agents, a number of issues will make the approval of biosimilars more complicated. Documents recently published by the European Medicines Agency (EMEA) outlining requirements for the market approval of biosimilars provide much-needed guidance. The EMEA has approved a number of biosimilar products in a scientifically rigorous and balanced process. Outstanding issues include the interchangeability of biosimilars and innovator products, the possible need for unique naming to differentiate the various biopharmaceutical products, and more comprehensive labelling for biosimilars to include relevant clinical data. 5
Biosimilars: policy, clinical, and regulatory considerations.
The regulatory background surrounding biosimilars (biopharmaceuticals that are considered similar in composition to an innovator product, but not necessarily clinically interchangeable); equivalence, interchangeability, and unique considerations associated with biopharmaceuticals; the biopharmaceutical protein production process; scientific facts for use in the policy discussion about biosimilars; the European Union system for biosimilars; and the current status of biosimilars legislation in the United States are described.
An abbreviated regulatory pathway for the approval of biosimilars, and a process for safely demonstrating the therapeutic interchangeability of these proteins, has the potential to provide meaningful cost savings. This economic advantage to patients can translate into important public health benefits. But to date, no formal regulatory process exists in the United States for bringing these drugs to market. In addition, the current tools for fully characterizing biopharmaceuticals are not--in certain cases--well developed, especially for proteins that have complex structures or are heavily glycosylated. In addition, using "similar" but not completely "identical" proteins interchangeably raises concerns about potentiating immunogenicity. The bottom line is that demonstrating therapeutic equivalence and interchangeability for biosimilars is not a straightforward matter--it cannot be based on the same criteria as for conventional small-molecule drugs. The science, while obtainable, is more complex. For example, it is assumed that showing that a biosimilar protein can be safely used interchangeably with an innovator protein would require, at the least, some limited clinical data and interchangeability studies. Notwithstanding the more complex scientific and clinical issues particular to protein products, most believe that a process for enabling the approval of safe and effective biosimilar proteins is not only possible, but an important public health goal. The European Union system for biosimilars may provide a model for anticipating and resolving the scientific and policy issues related to biosimilars in the U.S.
The legal and regulatory status of biosimilars remains to be resolved in the United States as policymakers address the scientific and policy issues surrounding product manufacturing, patent terms, and clinical use.
Biosimilars: it's not as simple as cost alone.
Biosimilars or follow-on biologics (FoB) are biopharmaceuticals that, unlike small molecule generic products, are copies of larger, much more complex proteins. As such, data generated from one biopharmaceutical cannot be extrapolated to another. Unlike small molecule generics, FoB require a full developmental programme, albeit smaller than for an originator product. This has been recognized by European regulatory authorities and it is becoming clear that accelerated processes for FoB marketing approval are not feasible.
To determine the balance between costs surrounding FoB (including relatively extensive developmental programmes and subsequent price to the market) and the necessity to ensure efficacy and safety.
It is important that FoB are sufficiently tested to ensure patient safety is not compromised. Conducting such a development programme followed by sound pharmacovigilance is very challenging and costly.
Cost-savings associated with FoB may be limited. 10
Recommendations regarding technical standards for follow-on biologics: comparability, similarity, interchangeability.
Policy makers around the world are currently considering the creation of a regulatory pathway for follow-on biologics (FOB), which will have to account for the substantial technical challenges associated with FOB development. These challenges will likely involve more complexity than comparability assessments of process changes made by the same manufacturer. The history of industry-regulator comparability discussions helps explain why the same degree of testing and flexibility now applied to change-control within a manufacturer's own process, at this time, cannot be extrapolated to the observed and possibly unknown differences between two manufacturing processes that are independently developed by different (non-collaborating) parties.
This commentary provides recommendations on the technical aspects that should be considered in the creation of an approval pathway for FOB products.
In the authors' view, analytical methodology in its current state cannot alone provide full assurance that the FOB is sufficiently similar to the innovator product. Moreover, the FOB manufacturer will not have access to the extensive knowledge accumulated by the innovator manufacturer from early development through marketing. Thus, extensive clinical evaluation will likely be necessary to provide assurance that the FOB is safe and efficacious. If such testing demonstrates the FOB is safe and efficacious per existing regulatory standards, the product should receive marketing approval as a 'similar' product. Since 'similarity' is a fundamentally different determination than establishing interchangeability between the two products, an interchangeability determination must be based on additional testing and market experience to ensure patient safety. Post-marketing surveillance of the FOB should be conducted to ensure that the approved molecule has similar clinical safety and efficacy as the innovator product, prior to any consideration of interchangeability 11
European regulatory guidelines for biosimilars.
The impending arrival en masse of biosimilars on Western markets is placing drug regulatory agencies under pressure to realign their policies. Biosimilars require more rigorous assessments than traditional chemical generics. This is because of the molecular complexity of recombinant proteins, and the complexity of biological manufacturing processes. Small differences can arise in a recombinant protein product which are hard or impossible to detect with even state-of-the-art analytical techniques. Yet, these differences can have significant impact on the safety and efficacy of the drug. The European Medicines Agency (EMEA) has taken the lead in issuing guidelines, most of which are still under review. The guidelines advocate pre-clinical and clinical testing of biosimilars prior to market authorization, complemented by tailored pharmacovigilance plans. These guidelines provide a valuable base from which to develop in this evolving regulatory environment.12
Legislative initiatives in Europe, Canada and the US for market authorization of follow-on biologics.
Legislative initiatives in Europe, Canada and the US for market authorization of follow-on biologics.
The formulation and application of legal and regulatory requirements for the market authorization of follow-on versions of biological drugs present challenges. This review discusses relevant regulatory guidelines and legislative initiatives related to market authorization for follow-on biologics in Europe, Canada and the US. The respective positions of these three markets is analyzed with regard to several factors: criteria for the choice of reference products; requirements for the comparability exercise between a candidate follow-on biologic and the selected reference product, with an emphasis on considerations of quality, safety and efficacy data; the interchangeability of a reference product with related follow-on drugs; data exclusivity provisions; and the application of specialized patent enforcement mechanisms to follow-on biologics.13
Quality, safety and efficacy of follow-on biologics in Japan.
Recently, WHO, EU, Japan and Canada have published guidelines on biosimilar/follow-on biologics. While there seems to be no significant difference in the general concept in these guidelines, the data to be submitted for product approval are partially different. Differences have been noted in the requirements for comparability studies on stability, prerequisites for reference product, or for the need of comparability exercise for determination of process-related impurities. In Japan, there have been many discussions about the amount and extent of data for approval of follow-on biologics. We try to clarify the scientific background and rational for regulatory pathway of biosimilar/follow-on biologics in Japan in comparison with the guidelines available from WHO, EU and Canada. In this article, we address and discuss the scientific background underlying these differences to facilitate the harmonization of follow-on biologic principles in the guidelines in future.14
References
1. www.fda.gov. -Generic drugs, fact sheethttp://wwwfdagov/Drugs/ResourcesForYou/Consumers/Buying Using Medicine SafelyUnderstandingGenericDrugs/ucm167991.htm .
Subscribe to:
Comments (Atom)


