 ASPARAGUS
ASPARAGUS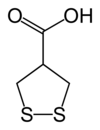
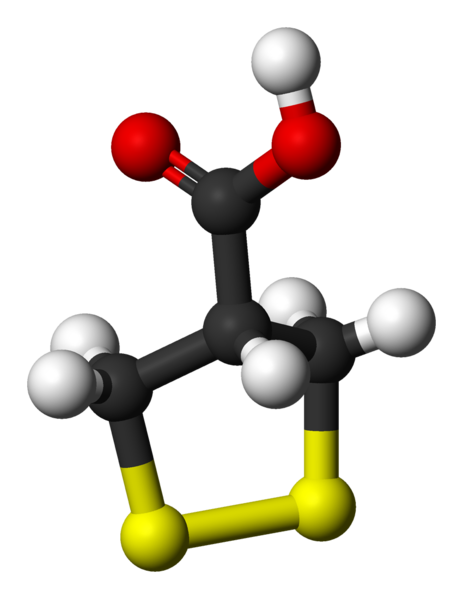
Asparagusic acid

Asparagusic acid is the organosulfur with the formula S2(CH2)2CHCO2H. The molecule contains both carboxylic acid and disulfide functional groups. It is present in the vegetable asparagus and may be the metabolic precursor to other odorous thiol compounds.
Biosynthetic studies revealed that asparagusic acid is derived from isobutyric acid. This colorless solid has a melting point (m.p.) of 75.7–76.5 °C. The corresponding dithiol (m.p. 59.5–60.5 °C) is also known; it is called dihydroasparagusic acid or dimercaptoisobutyric acid.
Over the past forty years several papers have been published on the subject, and several studies undertaken, to try and determine the chemical compounds responsible, and though there is still no definitive verdict as to the manner in which these compounds are formed, it has been suggested that they all form from asparagusic acid.

Asparagusic acid is, unsurprisingly considering the name, a chemical found exclusively in asparagus, and absent in other related vegetables.
The asparagus-pee molecules that you smell come mostly from the breakdown of a molecule known as asparagusic acid, which is present naturally in asparagus. When your body breaks down asparagusic acid it forms a wide variety of chemicals, all of which contain sulfur!

This has made it an obvious candidate for being the origin of the peculiar effect that asparagus has on urine. It has been suggested by recent studies that it could be metabolised in the body to produce the volatile compounds found in the urine after consuming the vegetable.
Steamed asparagus prepared with roasted pine nuts
Many chemicals that contain sulfur atoms smell horrible in similar ways, and I have no idea why this is. This is one chemical/biological mystery that, much to my chagrin, remains unsolved in my head (internet people, if the reason is known, please help!).
Aside from sulfur, the thing that all these smelly asparagus-pee chemicals have in common is that they are “light” enough (a.k.a. they are “volatile”, which means they have a relatively low boiling point) that they can float up into the air and into your nose. That is partly why asparagus doesn’t smell like asparagus-pee, because asparagusic acid is not volatile (remember that word). In fact, asparagusic acid boils above 300 °C (>600 °F), so there is no way any of it gets into your nose!
Asparagus has been used as a vegetable and medicine, owing to its delicate flavour, diuretic properties, and more. It is pictured as an offering on an Egyptian frieze dating to 3000 BC. Still in ancient times, it was known in Syria and in Spain. Greeks and Romans ate it fresh when in season and dried the vegetable for use in winter; Romans would even freeze it high in the Alps, for the Feast of Epicurus. Emperor Augustus tossed off the “Asparagus Fleet” for hauling the vegetable, and coined the expression “faster than cooking asparagus” for quick action. A recipefor cooking asparagus is in the oldest surviving book of recipes, Apicius’s third-century AD De re coquinaria, Book III.
The ancient Greek physician Galen (prominent among the Romans) mentioned asparagus as a beneficial herb during the second century AD, but after the Roman empire ended, asparagus drew little medieval attention. until al-Nafzawi‘s The Perfumed Garden. That piece of writing celebrates its (scientifically unconfirmed) aphrodisiacal power, a supposed virtue that the IndianAnanga Ranga attributes to “special phosphorus elements” that also counteract fatigue. By 1469, asparagus was cultivated in French monasteries. Asparagus appears to have been hardly noticed in England until 1538, and in Germany until 1542.
The finest texture and the strongest and yet most delicate taste is in the tips. The points d’amour (“love tips”) were served as a delicacy to Madame de Pompadour. Asparagus became available to the New World around 1850, in the United States.
Chemistry
Certain compounds in asparagus are metabolized to yield ammonia and various sulfur-containing degradation products, including various thiols andthioesters, which give urine a characteristic smell.
Some of the volatile organic compounds responsible for the smell are:
- methanethiol
- dimethyl sulfide
- dimethyl disulfide
- bis(methylthio)methane
- dimethyl sulfoxide
- dimethyl sulfone
Subjectively, the first two are the most pungent, while the last two (sulfur-oxidized) give a sweet aroma. A mixture of these compounds form a “reconstituted asparagus urine” odor. This was first investigated in 1891 by Marceli Nencki, who attributed the smell to methanethiol. These compounds originate in the asparagus as asparagusic acid and its derivatives, as these are the only sulfur-containing compounds unique to asparagus. As these are more present in young asparagus, this accords with the observation that the smell is more pronounced after eating young asparagus. The biological mechanism for the production of these compounds is less clear.
The onset of the asparagus urine smell is remarkably rapid. The smell has been reported to be detectable 15 to 30 minutes after ingestion.
Gas chromatography-mass spectrometry was used to analyse the ‘headspace’ of urine produced after consumption of asparagus. The headspace is the gas space immediately above the liquid surface, which is occupied by light, volatile compounds in the liquid, and analysis of this is useful in identifying odour-causing compounds. The analysis of the post-asparagus urine showed the presence of several compounds that were not present, or present in negligible amounts, in normal urine. The primary compounds present, in quantities a thousand times greater than in normal urine, were methanethiol and dimethyl sulfide. The compounds dimethyl sulfide and dimethyl sulfone were also present and it was suggested that they modify the aroma to give it a ‘sweet’ edge.
| NUTRITIONAL VALUE PER 100 G (3.5 OZ) | |
|---|---|
| ENERGY | 85 kJ (20 kcal) |
| CARBOHYDRATES | 3.88 g |
| - SUGARS | 1.88 g |
| - DIETARY FIBRE | 2.1 g |
| FAT | 0.12 g |
| PROTEIN | 2.2 g |
| Vitamin A equiv. | 38 μg (5%) |
| - beta-carotene | 449 μg (4%) |
| - lutein and zeaxanthin | 710 μg |
| Thiamine (vit. B1) | 0.143 mg (12%) |
| Riboflavin (vit. B2) | 0.141 mg (12%) |
| Niacin (vit. B3) | 0.978 mg (7%) |
| Pantothenic acid (B5) | 0.274 mg (5%) |
| Vitamin B6 | 0.091 mg (7%) |
| Folate (vit. B9) | 52 μg (13%) |
| Choline | 16 mg (3%) |
| Vitamin C | 5.6 mg (7%) |
| Vitamin E | 1.1 mg (7%) |
| Vitamin K | 41.6 μg (40%) |
| Calcium | 24 mg (2%) |
| Iron | 2.14 mg (16%) |
| Magnesium | 14 mg (4%) |
| Manganese | 0.158 mg (8%) |
| Phosphorus | 52 mg (7%) |
| Potassium | 202 mg (4%) |
| Sodium | 2 mg (0%) |
| Zinc | 0.54 mg (6%) |
| Link to USDA Database entry Percentages are roughly approximated using US recommendations for adults. Source: USDA Nutrient Database | |



No comments:
Post a Comment