
Thursday, August 21, 2014 - Bristol-Myers Squibb Company (NYSE: BMY) and Pfizer Inc. (NYSE: PFE) today announced the U.S. Food and Drug Administration (FDA) has approved a Supplemental New Drug Application (sNDA) for Eliquis for the treatment of DVT and PE, and for the reduction in the risk of recurrent DVT and PE following initial therapy. Combined, DVT and PE are known as VTE. It is estimated that every year, approximately 900,000 Americans are affected by DVT and PE.
http://www.drugs.com/newdrugs/fda-approves-eliquis-apixaban-deep-vein-thrombosis-pulmonary-embolism-4073.html?utm_source=ddc&utm_medium=email&utm_campaign=Today%27s+news+summary+-+August+21%2C+2014
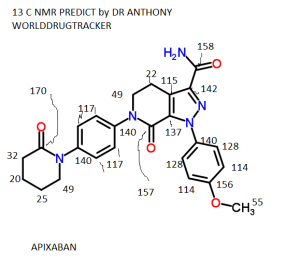
Bristol-Myers Squibb Company and Pfizer Inc. announced
the US Food and Drug Administration (FDA) has approved a Supplemental
New Drug Application (sNDA) for Eliquis (apixaban) for the treatment of
deep vein thrombosis (DVT) and pulmonary embolism (PE). The FDA also
approved an expanded use for Eliquis reducing the risk of recurrent DVT
and PE following initial therapy.
The full Prescribing Information for Eliquis includes Boxed Warnings for the increased risk of thrombotic events in patients who prematurely discontinue Eliquis; and for the increased risk of epidural or spinal hematoma, which may cause long-term or permanent paralysis, in patients using Eliquis and undergoing spinal epidural anesthesia or spinal puncture.
Eliquis increases the risk of bleeding and can cause serious, potentially fatal, bleeding.
Eliquis was approved for the treatment of DVT and PE, and for the reduction in the risk of recurrent DVT and PE following initial therapy, based on data from the global AMPLIFY and AMPLIFY-EXT studies.
The AMPLIFY study, a randomized, double-blind trial, was designed to demonstrate the efficacy and safety of Eliquis for the treatment of DVT and PE, and included patients with confirmed symptomatic DVT or PE (2,609 for Eliquis and 2,635 for standard of care, which was initial enoxaparin treatment for at least five days, overlapped by warfarin therapy [International Normalized Ratio (INR) range 2.0-3.0] orally for six months).
In the AMPLIFY study, Eliquis 10 mg twice daily for one week followed by 5 mg twice daily for six months demonstrated efficacy comparable to standard of care in treating DVT and PE patients for the primary efficacy composite endpoint of recurrent, symptomatic VTE, or VTE-related death (2.3% vs. 2.7%, relative risk, 0.84; 95% confidence interval [CI], 0.60 to 1.18; P-value<0.0001 for noninferiority).
Eliquis demonstrated superiority in the primary safety endpoint of major bleeding versus standard of care (0.6% vs. 1.8%, relative risk 0.31; 95% CI, 0.17 to 0.55; P<0.0001 for superiority). Major bleeding was defined as clinically overt bleeding that was accompanied by one or more of the following: a decrease in the hemoglobin level of 2 g/dL or more; a transfusion of two or more units of packed red blood cells; bleeding that occurred in at least one of the following critical sites: intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal; or bleeding that was fatal.
For the secondary safety endpoint in the AMPLIFY study, the event rates for clinically relevant nonmajor bleeding (CRNM) were fewer in Eliquis-treated patients compared to standard of care-treated patients (3.9% vs. 8.0%). CRNM was defined as overt bleeding that did not meet the criteria for major bleeding but was associated with a medical intervention, contact with a physician, interruption of the study drug, or discomfort or impairment in carrying out daily activities.
In AMPLIFY, the discontinuation rate due to bleeding events was 0.7% in the Eliquis-treated patients compared to 1.7% in enoxaparin/warfarin-treated patients.
“We are pleased that Eliquis is now available as an effective treatment option for DVT and PE,” said Douglas Manion, MD, Head of Specialty Development, Bristol-Myers Squibb. “Eliquis offers oral dosing, no routine coagulation testing, and does not require the use of a parenteral anticoagulant or bridging during initiation.”
- See more at: http://www.hcplive.com/product-news/FDA-Approves-Eliquis-apixaban-for-the-Treatment-of-Deep-Vein-Thrombosis-and-Pulmonary-Embolism#sthash.v2fo89oO.dpuf
The full Prescribing Information for Eliquis includes Boxed Warnings for the increased risk of thrombotic events in patients who prematurely discontinue Eliquis; and for the increased risk of epidural or spinal hematoma, which may cause long-term or permanent paralysis, in patients using Eliquis and undergoing spinal epidural anesthesia or spinal puncture.
Eliquis increases the risk of bleeding and can cause serious, potentially fatal, bleeding.
Eliquis was approved for the treatment of DVT and PE, and for the reduction in the risk of recurrent DVT and PE following initial therapy, based on data from the global AMPLIFY and AMPLIFY-EXT studies.
The AMPLIFY study, a randomized, double-blind trial, was designed to demonstrate the efficacy and safety of Eliquis for the treatment of DVT and PE, and included patients with confirmed symptomatic DVT or PE (2,609 for Eliquis and 2,635 for standard of care, which was initial enoxaparin treatment for at least five days, overlapped by warfarin therapy [International Normalized Ratio (INR) range 2.0-3.0] orally for six months).
In the AMPLIFY study, Eliquis 10 mg twice daily for one week followed by 5 mg twice daily for six months demonstrated efficacy comparable to standard of care in treating DVT and PE patients for the primary efficacy composite endpoint of recurrent, symptomatic VTE, or VTE-related death (2.3% vs. 2.7%, relative risk, 0.84; 95% confidence interval [CI], 0.60 to 1.18; P-value<0.0001 for noninferiority).
Eliquis demonstrated superiority in the primary safety endpoint of major bleeding versus standard of care (0.6% vs. 1.8%, relative risk 0.31; 95% CI, 0.17 to 0.55; P<0.0001 for superiority). Major bleeding was defined as clinically overt bleeding that was accompanied by one or more of the following: a decrease in the hemoglobin level of 2 g/dL or more; a transfusion of two or more units of packed red blood cells; bleeding that occurred in at least one of the following critical sites: intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal; or bleeding that was fatal.
For the secondary safety endpoint in the AMPLIFY study, the event rates for clinically relevant nonmajor bleeding (CRNM) were fewer in Eliquis-treated patients compared to standard of care-treated patients (3.9% vs. 8.0%). CRNM was defined as overt bleeding that did not meet the criteria for major bleeding but was associated with a medical intervention, contact with a physician, interruption of the study drug, or discomfort or impairment in carrying out daily activities.
In AMPLIFY, the discontinuation rate due to bleeding events was 0.7% in the Eliquis-treated patients compared to 1.7% in enoxaparin/warfarin-treated patients.
“We are pleased that Eliquis is now available as an effective treatment option for DVT and PE,” said Douglas Manion, MD, Head of Specialty Development, Bristol-Myers Squibb. “Eliquis offers oral dosing, no routine coagulation testing, and does not require the use of a parenteral anticoagulant or bridging during initiation.”
- See more at: http://www.hcplive.com/product-news/FDA-Approves-Eliquis-apixaban-for-the-Treatment-of-Deep-Vein-Thrombosis-and-Pulmonary-Embolism#sthash.v2fo89oO.dpuf
Bristol-Myers Squibb Company and Pfizer Inc. announced
the US Food and Drug Administration (FDA) has approved a Supplemental
New Drug Application (sNDA) for Eliquis (apixaban) for the treatment of
deep vein thrombosis (DVT) and pulmonary embolism (PE). The FDA also
approved an expanded use for Eliquis reducing the risk of recurrent DVT
and PE following initial therapy.
The full Prescribing Information for Eliquis includes Boxed Warnings for the increased risk of thrombotic events in patients who prematurely discontinue Eliquis; and for the increased risk of epidural or spinal hematoma, which may cause long-term or permanent paralysis, in patients using Eliquis and undergoing spinal epidural anesthesia or spinal puncture.
Eliquis increases the risk of bleeding and can cause serious, potentially fatal, bleeding.
Eliquis was approved for the treatment of DVT and PE, and for the reduction in the risk of recurrent DVT and PE following initial therapy, based on data from the global AMPLIFY and AMPLIFY-EXT studies.
The AMPLIFY study, a randomized, double-blind trial, was designed to demonstrate the efficacy and safety of Eliquis for the treatment of DVT and PE, and included patients with confirmed symptomatic DVT or PE (2,609 for Eliquis and 2,635 for standard of care, which was initial enoxaparin treatment for at least five days, overlapped by warfarin therapy [International Normalized Ratio (INR) range 2.0-3.0] orally for six months).
In the AMPLIFY study, Eliquis 10 mg twice daily for one week followed by 5 mg twice daily for six months demonstrated efficacy comparable to standard of care in treating DVT and PE patients for the primary efficacy composite endpoint of recurrent, symptomatic VTE, or VTE-related death (2.3% vs. 2.7%, relative risk, 0.84; 95% confidence interval [CI], 0.60 to 1.18; P-value<0.0001 for noninferiority).
Eliquis demonstrated superiority in the primary safety endpoint of major bleeding versus standard of care (0.6% vs. 1.8%, relative risk 0.31; 95% CI, 0.17 to 0.55; P<0.0001 for superiority). Major bleeding was defined as clinically overt bleeding that was accompanied by one or more of the following: a decrease in the hemoglobin level of 2 g/dL or more; a transfusion of two or more units of packed red blood cells; bleeding that occurred in at least one of the following critical sites: intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal; or bleeding that was fatal.
For the secondary safety endpoint in the AMPLIFY study, the event rates for clinically relevant nonmajor bleeding (CRNM) were fewer in Eliquis-treated patients compared to standard of care-treated patients (3.9% vs. 8.0%). CRNM was defined as overt bleeding that did not meet the criteria for major bleeding but was associated with a medical intervention, contact with a physician, interruption of the study drug, or discomfort or impairment in carrying out daily activities.
In AMPLIFY, the discontinuation rate due to bleeding events was 0.7% in the Eliquis-treated patients compared to 1.7% in enoxaparin/warfarin-treated patients.
“We are pleased that Eliquis is now available as an effective treatment option for DVT and PE,” said Douglas Manion, MD, Head of Specialty Development, Bristol-Myers Squibb. “Eliquis offers oral dosing, no routine coagulation testing, and does not require the use of a parenteral anticoagulant or bridging during initiation.”
- See more at: http://www.hcplive.com/product-news/FDA-Approves-Eliquis-apixaban-for-the-Treatment-of-Deep-Vein-Thrombosis-and-Pulmonary-Embolism#sthash.v2fo89oO.dpuf
The full Prescribing Information for Eliquis includes Boxed Warnings for the increased risk of thrombotic events in patients who prematurely discontinue Eliquis; and for the increased risk of epidural or spinal hematoma, which may cause long-term or permanent paralysis, in patients using Eliquis and undergoing spinal epidural anesthesia or spinal puncture.
Eliquis increases the risk of bleeding and can cause serious, potentially fatal, bleeding.
Eliquis was approved for the treatment of DVT and PE, and for the reduction in the risk of recurrent DVT and PE following initial therapy, based on data from the global AMPLIFY and AMPLIFY-EXT studies.
The AMPLIFY study, a randomized, double-blind trial, was designed to demonstrate the efficacy and safety of Eliquis for the treatment of DVT and PE, and included patients with confirmed symptomatic DVT or PE (2,609 for Eliquis and 2,635 for standard of care, which was initial enoxaparin treatment for at least five days, overlapped by warfarin therapy [International Normalized Ratio (INR) range 2.0-3.0] orally for six months).
In the AMPLIFY study, Eliquis 10 mg twice daily for one week followed by 5 mg twice daily for six months demonstrated efficacy comparable to standard of care in treating DVT and PE patients for the primary efficacy composite endpoint of recurrent, symptomatic VTE, or VTE-related death (2.3% vs. 2.7%, relative risk, 0.84; 95% confidence interval [CI], 0.60 to 1.18; P-value<0.0001 for noninferiority).
Eliquis demonstrated superiority in the primary safety endpoint of major bleeding versus standard of care (0.6% vs. 1.8%, relative risk 0.31; 95% CI, 0.17 to 0.55; P<0.0001 for superiority). Major bleeding was defined as clinically overt bleeding that was accompanied by one or more of the following: a decrease in the hemoglobin level of 2 g/dL or more; a transfusion of two or more units of packed red blood cells; bleeding that occurred in at least one of the following critical sites: intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal; or bleeding that was fatal.
For the secondary safety endpoint in the AMPLIFY study, the event rates for clinically relevant nonmajor bleeding (CRNM) were fewer in Eliquis-treated patients compared to standard of care-treated patients (3.9% vs. 8.0%). CRNM was defined as overt bleeding that did not meet the criteria for major bleeding but was associated with a medical intervention, contact with a physician, interruption of the study drug, or discomfort or impairment in carrying out daily activities.
In AMPLIFY, the discontinuation rate due to bleeding events was 0.7% in the Eliquis-treated patients compared to 1.7% in enoxaparin/warfarin-treated patients.
“We are pleased that Eliquis is now available as an effective treatment option for DVT and PE,” said Douglas Manion, MD, Head of Specialty Development, Bristol-Myers Squibb. “Eliquis offers oral dosing, no routine coagulation testing, and does not require the use of a parenteral anticoagulant or bridging during initiation.”
- See more at: http://www.hcplive.com/product-news/FDA-Approves-Eliquis-apixaban-for-the-Treatment-of-Deep-Vein-Thrombosis-and-Pulmonary-Embolism#sthash.v2fo89oO.dpuf
Bristol-Myers Squibb Company and Pfizer Inc. announced
the US Food and Drug Administration (FDA) has approved a Supplemental
New Drug Application (sNDA) for Eliquis (apixaban) for the treatment of
deep vein thrombosis (DVT) and pulmonary embolism (PE). The FDA also
approved an expanded use for Eliquis reducing the risk of recurrent DVT
and PE following initial therapy.
The full Prescribing Information for Eliquis includes Boxed Warnings for the increased risk of thrombotic events in patients who prematurely discontinue Eliquis; and for the increased risk of epidural or spinal hematoma, which may cause long-term or permanent paralysis, in patients using Eliquis and undergoing spinal epidural anesthesia or spinal puncture.
Eliquis increases the risk of bleeding and can cause serious, potentially fatal, bleeding.
Eliquis was approved for the treatment of DVT and PE, and for the reduction in the risk of recurrent DVT and PE following initial therapy, based on data from the global AMPLIFY and AMPLIFY-EXT studies.
The AMPLIFY study, a randomized, double-blind trial, was designed to demonstrate the efficacy and safety of Eliquis for the treatment of DVT and PE, and included patients with confirmed symptomatic DVT or PE (2,609 for Eliquis and 2,635 for standard of care, which was initial enoxaparin treatment for at least five days, overlapped by warfarin therapy [International Normalized Ratio (INR) range 2.0-3.0] orally for six months).
In the AMPLIFY study, Eliquis 10 mg twice daily for one week followed by 5 mg twice daily for six months demonstrated efficacy comparable to standard of care in treating DVT and PE patients for the primary efficacy composite endpoint of recurrent, symptomatic VTE, or VTE-related death (2.3% vs. 2.7%, relative risk, 0.84; 95% confidence interval [CI], 0.60 to 1.18; P-value<0.0001 for noninferiority).
Eliquis demonstrated superiority in the primary safety endpoint of major bleeding versus standard of care (0.6% vs. 1.8%, relative risk 0.31; 95% CI, 0.17 to 0.55; P<0.0001 for superiority). Major bleeding was defined as clinically overt bleeding that was accompanied by one or more of the following: a decrease in the hemoglobin level of 2 g/dL or more; a transfusion of two or more units of packed red blood cells; bleeding that occurred in at least one of the following critical sites: intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal; or bleeding that was fatal.
For the secondary safety endpoint in the AMPLIFY study, the event rates for clinically relevant nonmajor bleeding (CRNM) were fewer in Eliquis-treated patients compared to standard of care-treated patients (3.9% vs. 8.0%). CRNM was defined as overt bleeding that did not meet the criteria for major bleeding but was associated with a medical intervention, contact with a physician, interruption of the study drug, or discomfort or impairment in carrying out daily activities.
In AMPLIFY, the discontinuation rate due to bleeding events was 0.7% in the Eliquis-treated patients compared to 1.7% in enoxaparin/warfarin-treated patients.
“We are pleased that Eliquis is now available as an effective treatment option for DVT and PE,” said Douglas Manion, MD, Head of Specialty Development, Bristol-Myers Squibb. “Eliquis offers oral dosing, no routine coagulation testing, and does not require the use of a parenteral anticoagulant or bridging during initiation.”
- See more at: http://www.hcplive.com/product-news/FDA-Approves-Eliquis-apixaban-for-the-Treatment-of-Deep-Vein-Thrombosis-and-Pulmonary-Embolism#sthash.v2fo89oO.dpuf
The full Prescribing Information for Eliquis includes Boxed Warnings for the increased risk of thrombotic events in patients who prematurely discontinue Eliquis; and for the increased risk of epidural or spinal hematoma, which may cause long-term or permanent paralysis, in patients using Eliquis and undergoing spinal epidural anesthesia or spinal puncture.
Eliquis increases the risk of bleeding and can cause serious, potentially fatal, bleeding.
Eliquis was approved for the treatment of DVT and PE, and for the reduction in the risk of recurrent DVT and PE following initial therapy, based on data from the global AMPLIFY and AMPLIFY-EXT studies.
The AMPLIFY study, a randomized, double-blind trial, was designed to demonstrate the efficacy and safety of Eliquis for the treatment of DVT and PE, and included patients with confirmed symptomatic DVT or PE (2,609 for Eliquis and 2,635 for standard of care, which was initial enoxaparin treatment for at least five days, overlapped by warfarin therapy [International Normalized Ratio (INR) range 2.0-3.0] orally for six months).
In the AMPLIFY study, Eliquis 10 mg twice daily for one week followed by 5 mg twice daily for six months demonstrated efficacy comparable to standard of care in treating DVT and PE patients for the primary efficacy composite endpoint of recurrent, symptomatic VTE, or VTE-related death (2.3% vs. 2.7%, relative risk, 0.84; 95% confidence interval [CI], 0.60 to 1.18; P-value<0.0001 for noninferiority).
Eliquis demonstrated superiority in the primary safety endpoint of major bleeding versus standard of care (0.6% vs. 1.8%, relative risk 0.31; 95% CI, 0.17 to 0.55; P<0.0001 for superiority). Major bleeding was defined as clinically overt bleeding that was accompanied by one or more of the following: a decrease in the hemoglobin level of 2 g/dL or more; a transfusion of two or more units of packed red blood cells; bleeding that occurred in at least one of the following critical sites: intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal; or bleeding that was fatal.
For the secondary safety endpoint in the AMPLIFY study, the event rates for clinically relevant nonmajor bleeding (CRNM) were fewer in Eliquis-treated patients compared to standard of care-treated patients (3.9% vs. 8.0%). CRNM was defined as overt bleeding that did not meet the criteria for major bleeding but was associated with a medical intervention, contact with a physician, interruption of the study drug, or discomfort or impairment in carrying out daily activities.
In AMPLIFY, the discontinuation rate due to bleeding events was 0.7% in the Eliquis-treated patients compared to 1.7% in enoxaparin/warfarin-treated patients.
“We are pleased that Eliquis is now available as an effective treatment option for DVT and PE,” said Douglas Manion, MD, Head of Specialty Development, Bristol-Myers Squibb. “Eliquis offers oral dosing, no routine coagulation testing, and does not require the use of a parenteral anticoagulant or bridging during initiation.”
- See more at: http://www.hcplive.com/product-news/FDA-Approves-Eliquis-apixaban-for-the-Treatment-of-Deep-Vein-Thrombosis-and-Pulmonary-Embolism#sthash.v2fo89oO.dpuf
No comments:
Post a Comment