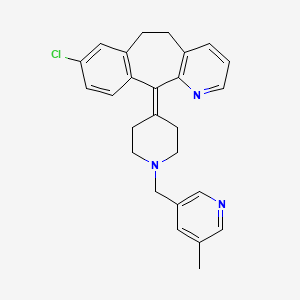
Rupatadine

Rupatadine
CAS 158876-82-5,
8-Chloro-11-(1-((5-methylpyridin-3-yl)methyl)piperidin-4-ylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine,
8-chloro-11-(1-[(5-methyl-3-pyridyl)methyl]-4-piperidyliden)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin
UNII-2AE8M83G3E, UR 12592
Molecular Formula: C26H26ClN3
Molecular Weight: 415.95774 g/mol
Percent Composition: C 75.07%, H 6.30%, Cl 8.52%, N 10.10%
Properties: Creamy solid, mp 58-61°.
Melting point: mp 58-61°
Platelet activating factor receptor antagonist; Histamine H1 receptor antagonist
Allergic rhinitis; Urticaria
| J. Uriach & Cia. S.A. |
Uriach developed and launched rupatadine for treating of allergic rhinitis and urticaria. Family members of the product case EP577957, have SPC protection in the EU until 2016.
As of January 2015, Newport Premium™ reports that Cadila Pharmaceuticals is producing or capable of producing commercial quantities of rupatadine fumarate and holds an active USDMF since September 2012.
Rupatadine is a second generation antihistamine and PAF antagonist used to treat allergies. It was discovered and developed by J. Uriach y Cia, S. A.[1] and is marketed under several trade names such as Rupafin, Alergoliber, Rinialer, Pafinur, Rupax and Ralif.
Therapeutic indications approved
Rupatadine fumarate has been approved for the treatment of allergic rhinitis and chronic urticaria in adults and children over 12 years. The defined daily dose (DDD) is 10 mg orally.

Derivative Type: Fumarate
CAS Registry Number: 182349-12-8
Trademarks: Rupafin (Uriach)
Molecular Formula: C26H26ClN3.C4H4O4
Molecular Weight: 532.03
Percent Composition: C 67.73%, H 5.68%, Cl 6.66%, N 7.90%, O 12.03%
Derivative Type: Trihydrochloride
CAS Registry Number: 156611-76-6
Molecular Formula: C26H26ClN3.3HCl
Molecular Weight: 525.34
Percent Composition: C 59.44%, H 5.56%, Cl 26.99%, N 8.00%
Properties: Crystals from ethyl acetate + ether, mp 213-217°.
Melting point: mp 213-217°.
Therap-Cat: Antihistaminic.
Keywords: Antihistaminic; Tricyclics; Other Tricyclics; Platelet Activating Factor Antagonist.
Available form
Rupatadine is available as round, light salmon coloured tablets containing 10 mg of rupatadine (as fumarate) to be administered orally, once a day.
Side effects
Rupatadine is a non-sedating antihistamine. However, as in other non sedating second-generation antihistamines, the most common side effects in controlled clinical studies weresomnolence, headaches and fatigue.
Mechanism of action
Rupatadine is a second generation, non-sedating, long-acting histamine antagonist with selective peripheral H1 receptor antagonist activity. It further blocks the receptors of the platelet-activating factor (PAF) according to in vitro and in vivo studies.[2]
Rupatadine possesses anti-allergic properties such as the inhibition of the degranulation ofmast cells induced by immunological and non-immunological stimuli, and inhibition of the release of cytokines, particularly of the TNF in human mast cells and monocytes.[3]

Pharmacokinetics
Rupatadine has several active metabolites such as desloratadine, 3-hydroxydesloratadine, 6-hydroxydesloratadine and 5-hydroxydesloratadine.
History
Rupatadine discovery, pre-clinical and clinical development was performed by J. Uriach y Cia, S. A., a Spanish pharmaceutical company. It was launched in 2003 in Spain by J. Uriach y Cia, S. A., with the brand name of Rupafin. The registration of the product is approved in 23 countries from the EU, 8 Central American countries, Brazil, Argentina, Chile, Turkey and 14 African countries.
Efficacy in humans
The efficacy of rupatadine as treatment for allergic rhinitis (AR) and chronic idiopathic urticaria (CIU) has been investigated in adults and adolescents (aged over 12 years) in several controlled studies, showing a rapid onset of action and a good safety profile even in prolonged treatment periods of a year.[3][4][5]
- Rupatadine is currently marketed in 10 mg (rupatadine) tablets as rupatadine fumarate (CAS 182349-12-8 for the fumarate salt) for the treatment of allergic rhinitis and urticaria in adults and teenagers.
- Rupatadine free base was first disclosed in EP0577957 .
- Spanish patent application ES2087818 discloses the monofumarate salt of rupatadine (i.e. rupatadine fumarate) and aqueous liquid pharmaceutical compositions of rupatadine fumarate. In particular, this document discloses a syrup containing rupatadine fumarate at 4 g/L, sucrose, a flavouring agent, a sweetening agent and water; and a solution for injection which contains rupatadine fumarate at 20 g/L, benzyl alcohol, propyleneglycol and water.
- Despite the aqueous liquid pharmaceutical compositions disclosed in EP0577957and ES2087818 , the inventors have found that the solubility in water of rupatadine fumarate is 2.9 g/L (see Reference example 1) and therefore these prior art formulations may have stability problems due to supersaturation of rupatadine free base or rupatadine fumarate and would not be suitable for use as a medicament.
- CN101669901 and CN101669926 disclose liquid formulations of rupatadine free base using cyclodextrins to dissolve rupatadine.
- CN101669901 is directed to liquid formulations of rupatadine free base for ophthalmic delivery comprising rupatadine, a solvent and a cyclodextrin.
- CN10169926 is directed to liquid formulations of rupatadine free base for nasal delivery comprising rupatadine, a solvent and a cyclodextrin. It is stated that rupatadine has low solubility in water (1.39 mg/mL to 0.82 mg/mL at pH 3.0 to 7.0, table 9 in CN10169926 ) and the problem of its low solubility is solved using cyclodextrins (tables 10-12 of CN10169926 ) in order to obtain liquid formulations.


The reaction of 2-cyano-3-methylpyridine (I) with H2SO4 in t-BuOH gives the N-tert-butylamide (II), which is treated with two equivalents of BuLi and the corresponding dianion alkylated with 3-chlorobenzyl chloride to afford amide (III). The treatment of (III) with POCl3 gives nitrile (IV) which is cyclized to ketone (V) by subsequent treatment with CF3SO3H and aqueous HCl. Reaction of ketone (V) with the Grignard derivative prepared from chloride (VI) affords alcohol (VII) which is finally dehydrated by H2SO4 to give UR-12592 (1), as shown in Scheme 20491401a. The key intermediate (VI) is synthesized through the condensation of 5-methylnicotinic acid (VIII) with 4-hydroxypiperidine by means of DCC in DMF to give amide (IX), followed by reduction with POCl3 and NaBH4 to give the amino alcohol (X) which is treated with SOCl2. Scheme 20491402a. Description White crystals, m.p. 196-8 癈. References 1. Carceller, E., Jim閚ez, P.J., Salas, J. (J. Uriach & Cia SA). Process for the preparation of 8-chloro-6,11-dihydro-11-[1-[(5-methyl-3-pyridinyl)methyl]-4 -piperidinylidene]-5H-benzo[5,6]cyclohepta[1,2-b]pyridine. ES 9602107.

The key intermediate (VI) is synthesized through the condensation of 5-methylnicotinic acid (VIII) with 4-hydroxypiperidine by means of DCC in DMF to give amide (IX), followed by reduction with POCl3 and NaBH4 to give the amino alcohol (X), which is treated with SOCl2.

………………………….
EXAMPLE 4
8-chloro-11-(1-[(5-methyl-3-pyridyl)methyl]-4-piperidyliden)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin
- To a solution of 1.7 mL (15 mmol) of 3,5-lutidine in 100 mL of CCl₄ was added 2.6 g (15 mmol) of NBS and the mixture was stirred at reflux under an argon atmosphere for 2 h. Then, the mixture was allowed to cool, the solid obtained was filtered off and to the filtrate was added 2.4 g (7.5 mmol) of the compound obtained in preparation 1 and 20 mg of 4-(dimethylamino)pyridine. The resulting mixture was stirred at room temperature for 18 h and 1.68 mL of triethylamine was added. It was diluted with 100 mL of dichloromethane and washed with 0.5N NaHCO₃ solution and with water. The organic phase was dried over sodium sulfate and the solvent was removed, to give 5.7 g of a residue that was chromatographed on silica gel (chloroform : methanol : ammonia, 60 : 2 : 0.2). 1.3 g of the title compound of the example was obtained as a white solid (yield: 40%).
mp: 58-61°C;
IR (KBr) ν: 3419, 3014, 1635, 1576, 1472 cm⁻¹;
¹H RMN (80 MHz, CDCl₃) δ (TMS): 8.39 (m, 3H, ar), 7.48 (m, 1H, ar), 7.37 (m, 1H, ar), 7.12 (m, 4H, ar), 3.45 (s, 2H, CH₂N), 3.36 (m, 2H), 3.1-2.1 (m, 13H). ¹³C RMN (20.15 MHz, CDCl₃) δ (TMS): 157.20 (C), 148.93 (CH), 147.46 (CH), 146.48 (CH), 139.50 (C), 138.56 (C), 137.06 (CH), 133.3 (C), 132.54 (C), 130.67 (CH), 128.80 (CH), 125.85 (CH), 121.92 (CH), 59.84 (CH₂), 54.63 (CH₂), 31.70 (CH₂), 31.32 (CH₂), 30.80 (CH₂), 30.56 (CH₂), 18.14 (CH₃).
………………………….
WO2006114676
Scheme-1
Example 1
Preparation of3-bromomethyl-5-methylpyridine hydrochloride: A mixture of carbon tetrachloride (4000ml), azobisisobutyronitrile (45.96gm, 0.279mol), 3,5-lutidine (150gm, 1.399mol) and N-bromosuccinamide (299.4gm, 1.682mol) is refluxed for 2 hours. The reaction mixture is cooled to room temperature and solid is filtered. HCl gas is passed to the filtrate and solid obtained is separated and filtered. Yield is 196gm Yield is 67.66%. Example 2
Preparation of Rupatadine :
A mixture of desloratadine (5.0gm, 0.016mol), methylene chloride (15ml), tetrabutylammonium bromide (0.575gm, 0.0018mol) and sodium hydroxide solution (2.5gm, 0.064mol in 8ml water) is cooled to 0 to 50C. 3-bromomethyl-5- methylpyridine hydrochloride (7.18gm, 0.032mol) in methylene chloride (35ml) is added to above mixture. The reaction mixture is stirred at 0 to 50C for 1 hour and at room temperature for 12 hours. Layers are separated and organic layer is washed with dilute HCl solution and water. Methylene chloride is distilled. Yield = 9.5g %Yield =
67.66%.
Example 3
Preparation of “Rupatadine fumarate:
A solution of fumaric acid (3.3gm) in methanol (46ml) is added to solution of
Rupatadine (4.5gm) in ethyl acetate (30ml) at room temperature. The reaction mass is cooled to -5 to O0C for 4 hours. Rupatadine fumarate is separated filtered and Washed with ethylacetate. Yield = 5.5 gm.
…………………………..
NEW PATENT
EP-02824103…An improved process for the preparation of rupatadine fumarate, Cadila Pharmaceuticals Ltd
Process for the preparing rupatadine intermediate (particularly 5-methylpyridine-3-methanol) comprises reduction of 5-methyl nicotinic acid alkyl ester using alkali metal borohydride is claimed. For a prior filing see WO2006114676, claiming the process for preparation of rupatadine fumarate.
……………………………………
J. Med. Chem., 1994, 37 (17), pp 2697–2703
DOI: 10.1021/jm00043a009
References
- Patents: EP 577957, US 5407941, US 5476856
- Merlos, M.; Giral, M.; Balsa, D.; Ferrando, R.; Queralt, M.; Puigdemont, A.; García-Rafanell, J.; Forn, J. (1997). “Rupatadine, a new potent, orally active dual antagonist of histamine and platelet-activating factor (PAF)”. The Journal of Pharmacology and Experimental Therapeutics 280 (1): 114–121. PMID 8996188.
- Picado, C. S. (2006). “Rupatadine: Pharmacological profile and its use in the treatment of allergic disorders”. Expert Opinion on Pharmacotherapy 7 (14): 1989–2001. doi:10.1517/14656566.7.14.1989. PMID 17020424.
- Keam, S. J.; Plosker, G. L. (2007). “Rupatadine: A review of its use in the management of allergic disorders”. Drugs 67 (3): 457–474. doi:10.2165/00003495-200767030-00008. PMID 17335300.
- Mullol, J.; Bousquet, J.; Bachert, C.; Canonica, W. G.; Gimenez-Arnau, A.; Kowalski, M. L.; Martí-Guadaño, E.; Maurer, M.; Picado, C.; Scadding, G.; Van Cauwenberge, P. (2008). “Rupatadine in allergic rhinitis and chronic urticaria”. Allergy 63: 5–28.doi:10.1111/j.1398-9995.2008.01640.x. PMID 18339040.
Literature References: Dual antagonist of histamine H1 and platelet-activating factor receptors. Prepn: E. Carceller et al., ES 2042421; eidem, US 5407941 (1993, 1995 both to Uriach);
eidem,J. Med. Chem. 37, 2697 (1994).
Mechanism of action: M. Merlos et al., J. Pharmacol. Exp. Ther. 280, 114 (1997).
Clinical trial in seasonal allergic rhinitis: F. Saint-Martin et al., J. Invest. Allergol. Clin. Immunol. 14, 34 (2004);
and comparison with ebastine: E. M. Guadaño et al., Allergy 59, 766 (2004).
Review of pharmacology and clinical development: N. Y. Van Den Anker-Rakhmanina, Curr. Opin. Anti-Inflam. Immunomod. Invest. Drugs 2, 127-132 (2000).
1 TO 8 OF 8
| ||
|---|---|---|
| PATENT | SUBMITTED | GRANTED |
| 8-chloro-11-[1-[(5-methyl-3-pyridyl)methyl]-4-piperidyliden]-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine [US5407941] | 1995-04-18 | |
| Treatment of PAF and histamine-mediated diseases with 8-chloro-11-[1-[(5-methyl-3-pyridyl)methyl]-4-piperidyliden]-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine [US5476856] | 1995-12-19 | |
| Process for the synthesis of n-(5-methylnicotinoyl)-4 hydroxypiperidine, a key intermediate of rupatadine [US6803468] | 2004-03-04 | 2004-10-12 |
| $g(b)2-ADRENERGIC RECEPTOR AGONISTS [EP1003540] | 2000-05-31 | |
| $g(b)2-ADRENERGIC RECEPTOR AGONISTS $g(b)2-ADRENERGIC RECEPTOR AGONISTS [EP1019360] | 2000-07-19 | |
| 8-Chloro-11-[1-[(5-methyl-3-pyridyl)methyl]-4-piperidyliden]-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine. [EP0577957] | 1994-01-12 | 1995-07-12 |
| NOVEL CRYSTALLINE FORM OF RUPATADINE FREE BASE [US2009197907] | 2009-08-06 | |
| METHODS FOR IDENTIFYING NOVEL MULTIMERIC AGENTS THAT MODULATE RECEPTORS METHODS FOR IDENTIFYING NOVEL MULTIMERIC AGENTS THAT MODULATE RECEPTORS [WO9966944] | 1999-12-29 | |
 | |
| SYSTEMATIC (IUPAC) NAME | |
|---|---|
| 8-Chloro-6,11-dihydro-11-[1-[(5-methyl-3-pyridinyl)methyl]-4-piperidinylidene]-5H-benzo[5,6]cyclohepta[1,2-b]pyridine fumarate | |
| CLINICAL DATA | |
| TRADE NAMES | Rupafin, Alergoliber, Rinialer, Pafinur, Rupax, Ralif |
| AHFS/DRUGS.COM | International Drug Names |
| LEGAL STATUS |
|
| ROUTES | Oral |
| PHARMACOKINETIC DATA | |
| PROTEIN BINDING | 98–99% |
| METABOLISM | Hepatic, CYP-mediated |
| HALF-LIFE | 5.9 hours |
| EXCRETION | 34.6% urine, 60.9% faeces |
| IDENTIFIERS | |
| CAS NUMBER | 158876-82-5 182349-12-8 (fumarate) |
| ATC CODE | R06AX28 |
| PUBCHEM | CID 133017 |
| CHEMSPIDER | 117388 |
| UNII | 2AE8M83G3E |
| CHEMBL | CHEMBL91397 |
| CHEMICAL DATA | |
| FORMULA | C26H26ClN3 |
| MOLECULAR MASS | 415.958 g/mol |


No comments:
Post a Comment