GSK-923295A
1088965-37-0
Synonym: GSK-923295; GSK 923295; GSK923295.
CENP-E Inhibitor
IUPAC/Chemical name:
3-Chloro-N-{(1S)-2-[(N,N-dimethylglycyl)amino]-1-[(4-{8-[(1S)-1-hydroxyethyl]imidazo[1,2-a]pyridin-2-yl}phenyl)methyl]ethyl}-4-[(1-methylethyl)oxy]benzamide
3-chloro-N-[(1S)-2-[[2-(dimethylamino)acetyl]amino]-1-[[4-[8-[(1S)-1-hydroxyethyl]imidazo[1,2-a]pyridin-2-yl]phenyl]methyl]ethyl]-4-(1-methylethoxy)- Benzamide,
3-Chloro-N-{(1S)-2-[(N,N-dimethylglycyl)amino]-1-[(4-{8-[(1S)-1-hydroxyethyl]imidazo[1,2-a]pyridin-2-yl}phenyl)methyl]ethyl}-4-[(1-methylethyl)oxy]benzamide
3-Chloro-N-[(1S)-2-[(N,N-dimethylglycyl)amino]-1-({4-[8-(1-hydroxyethyl)imidazo[1,2-a]pyridin-2-yl]phenyl}methyl)ethyl]-4-[(1-methylethyl)oxy]benzamide
3-Chloro-N-[1-(N,N-dimethylglycinamido)-3-[4-[8-[1(S)-hydroxyethyl]imidazo[1,2-a]pyridin-2-yl]phenyl]propan-2(S)-yl]-4-isopropoxybenzamide
C32H38ClN5O4
Exact Mass: 591.26123
Molecular Weight: 592.12822
Elemental Analysis: C, 64.91; H, 6.47; Cl, 5.99; N, 11.83; O, 10.81
Kinesin-like protein KIF11 inhibitor; Centromere protein E inhibitor
GSK-923295 is a novel antimitotic inhibitor of centromere-associated protein E (CENP-E) with potential anticancer activity. GSK923295A
demonstrated significant antitumor activity against solid tumor models,
inducing CRs in Ewing sarcoma, rhabdoid, and rhabdomyosarcoma
xenografts.
GSK-923295, a small-molecule inhibitor of centromere
associated protein (CENP), is in early clinical development at
Cytokinetics for the treatment of refractory cancer. No recent
development has been reported for early clinical research which had been
ongoing at GlaxoSmithKline.
Clinical study showed that
GSK923295 had
dose-proportional pharmacokinetics and a low number of grade 3 or 4
adverse events. The observed incidence of myelosuppression and
neuropathy was low. Further investigations may provide a more complete
understanding of the potential for
GSK923295 as an antiproliferative agent.
GSK923295 is a first-in-class, specific allosteric inhibitor of
CENP-E kinesin motor ATPase with
Ki of 3.2 nM, and less potent to mutant I182 and T183. Phase 1.
The
compound potently inhibits CENP-E ATPase activity and exerts
broad-spectrum antiproliferative activity against cancer cells and
xenografts. GSK-923295 has demonstrated a broad spectrum of activity
against a range of human tumor xenografts grown in nude mice, including
models of colon, breast, ovarian, lung and other tumors.
Cytokinetics
was developing GSK-923295, the lead from a series of small-molecule
mitotic kinesin spindle protein inhibitors, for treating cancer
including advanced solid tumors. However, since October 2014, the
program was no longer listed on the Cytokinetics' website
In 2001,
a strategic alliance was established between Cytokinetics and
GlaxoSmithKline to discover, develop and commercialize novel
small-molecule therapeutics targeting mitotic kinesins for applications
in the treatment of cancer and other diseases.
.........................
PATENT
US8772507
http://www.google.com/patents/US8772507
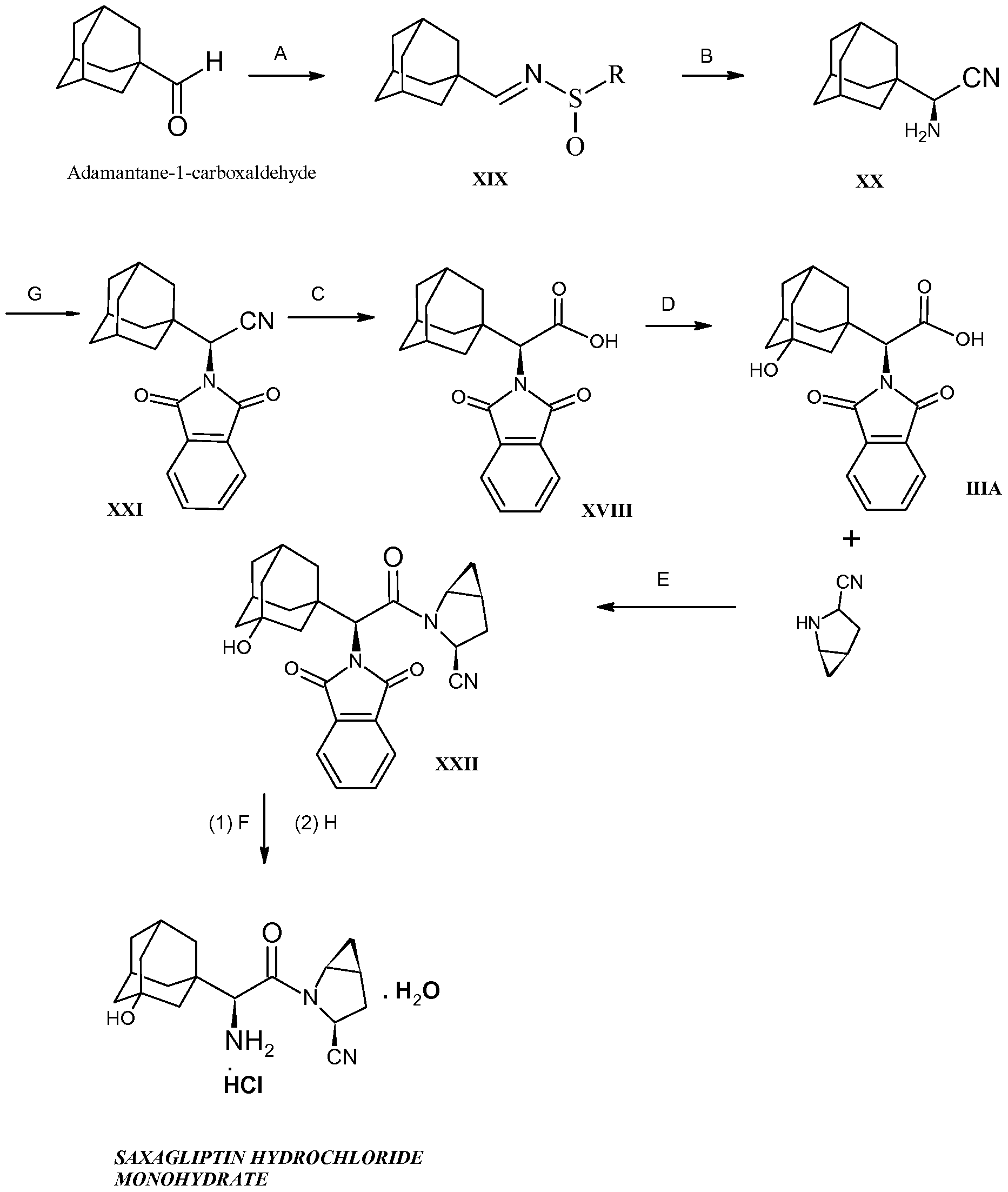
1,1-Dimethylethyl [(1S)-2-(4-bromophenyl)-1-(hydroxymethyl)ethyl]carbamate
To
a solution of
4-bromo-N-{[(1,1-dimethylethyl)oxy]carbonyl}-L-phenylalanine (72.6
mmol), in anhydrous diethyl ether (550 mL) at 0° C. was added slowly
lithium aluminum hydride, 95% (108.9 mmol). The resulting solution was
stirred for an additional 2 h at 0° C. The reaction was then carefully
quenched with a saturated aqueous solution of sodium bicarbonate (73 mL)
which stirred at RT for half an hour. Lithium aluminium salts crashed
out of solution and were removed by filtration. The filtrate was
concentrated and vacuum pumped for 24 h to afford the title product as a
white solid (97%). ESMS [M+H]
+: 331.2.
1,1-Dimethylethyl {(1S)-2-(4-bromophenyl)-1-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]ethyl}carbamate
To
a solution of 1,1-dimethylethyl
[(1S)-2-(4-bromophenyl)-1-(hydroxymethyl)ethyl]carbamate (70.6 mmol),
tripheylphosphine (84.7 mmol), and phthalimide (84.7 mmol) in anhydrous
tetrahydrofuran (550 mL) at 0° C. was added dropwise diisopropyl
azodicarboxylate (84.7 mmol) over 10 minutes. The reaction continued to
stir allowing to warm to RT over 5 h. The reaction was then concentrated
in vacuo and product was triturated out of solution using ethyl acetate
(500 mL). The precipitate was filtered, washed with ethyl acetate
(3×100 mL), and dried to afford the title product as a white solid
(57%). ESMS [M+H]
+: 460.4.
1,1-Dimethylethyl {(1S)-2-[4-(bromoacetyl)phenyl]-1-[(1,3-d oxo-1,3-dihydro-21′-isoindol-2-yl)methyl]ethyl}carbamate
A
solution of 1,1-dimethylethyl
{(1S)-2-(4-bromophenyl)-1-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]ethyl}carbamate
(21.7 mmol), 1-ethoxyvinyltri-n-butylin (43.5 mmol), and
trans-dichlorobis(triphenylphosphine)palladium(II) (5 mol %) were
stirred in anhydrous dioxane (300 mL) at 100° C. for 3 h. The reaction
was then concentrated in vacuo and redissolved in a solution of
tetrahydrofuran and water (3:1, 400 mL). The mixture was treated with
N-bromosuccinimide (108.8 mmol) and stirred at RT for half an hour. The
reaction solution was then concentrated to dryness and redissolved in
ethyl acetate (150 mL). Precipate formed upon addition of hexanes (350
mL) and was filtered and dried to afford the title product as yellow
solid (71%). ESMS [M+H]
+: 502.4.
1,1-Dimethylethyl
[(1S)-2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-1-({4-[8-(1-hydroxyethyl)imidazo[1,2-a]pyridin-2-yl]phenyl}methyl)ethyl]carbamate
A
mixture of
1,1-dimethylethyl{(1S)-2-{4-(bromoacetyl)phenyl]-1-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]ethyl}carbamate
(1.90 g, 3.79 mmol), 1-(2-amino-3-pyridinyl)ethanol (0.523 g, 3.79
mmol), and solid sodium bicarbonate (0.398 g, 4.72 mmol) in isopropanol
(24 mL) was refluxed for 3.0 h. The mixture was concentrated in vacuo
and the residue dissolved in ethyl acetate, washed with water and
saturated sodium chloride, dried (Na
2SO
4), and concentrated to give the title compound (1.79 g, 87%) as a light pink solid. MS (ES+) m/e 541 [M+H]
+.
3-Chloro-N-[(1S)-2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-1-({4-[8-(1-hydroxyethyl)imidazo[1,2-a]pyridin-2-yl]phenyl}methyl)ethyl]-4-[(1-methylethyl)oxy]benzamide
A
mixture of 1,1-dimethylethyl
[(1S)-2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-1-({4-[8-(1-hydroxyethyl)imidazo[1,2-a]pyridin-2-yl]phenyl}methyl)ethyl]carbamate
(1.79 g, 3.31 mmol) and 4 M HCl in 1,4-dioxane (20 mL, 80 mmol) was
stirred at room temperature for 45 minutes. The reaction was
concentrated to dryness and redissolved in DMF (30 mL). To this solution
was added N,N-diisopropylethylamine (2.14 g, 16.55 mmol) and
pentafluorophenyl 3-chloro-4 [(1-methylethyl)oxy]benzoate (1.36 g, 3.31
mmol). The mixture was stirred overnight at room temperature, diluted
with water, and extracted into ethyl acetate. The extracts were washed
with water, dried (Na
2SO
4), and concentrated in vacuo to give the title compound (2.10 g, 100%) as a tan solid. MS (ES+) m/e 637 [M+H]
+.
N-[(1S)-2-Amino-1-({4-[8-(1-hydroxyethyl)imidazo[1,2-a]pyridin-2-yl]phenyl}methyl)ethyl]-3-chloro-4-[(1-methylethyl)oxy]benzamide
A
mixture of
3-chloro-N-[(1S)-2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-1-({4-[8-(1-hydroxyethyl)imidazo[1,2-a]pyridin-2-yl]phenyl}methyl)ethyl]-4-[(1-methylethyl)oxy]benzamide
(2.10 g, 3.30 mmol) and hydrazine monohydrate (0.83 g, 16.5 mmol) in
ethanol (30 mL) was heated at 57° C. overnight. The reaction was cooled,
diluted with ethanol, filtered, and concentrated to give the title
compound (1.67 g, 100%) as a pale yellow powder. MS (ES+) m/e 507 [M+H]
+.
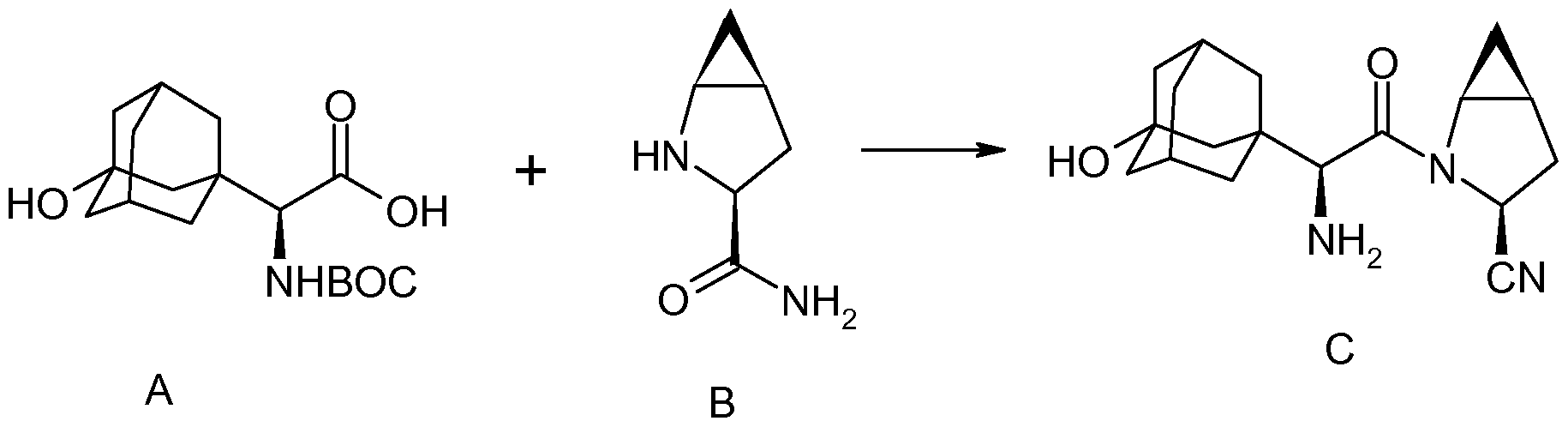
3-Chloro-N-[(1S)-2-[(N,N-dimethylglycyl)amino]-1-({4-[8-(1-hydroxyethyl)imidazo[1,2-a]pyridin-2-yl]phenyl}methyl)ethyl]-4-[(1-methylethyl)oxy]benzamide
A
mixture of
N-[(1S)-2-amino-1-({4-[8-(1-hydroxyethyl)imidazo[1,2-a]pyridin-2-yl]phenyl}methyl)ethyl]-3-chloro-4-[(1-methylethyl)oxy]benzamide
(0.912 g, 1.80 mmol), EDCI (0.69 g, 3.6 mmol),
N,N-diisopropylethylamine (0.466 g, 3.6 mmol), and N,N-dimethylglycine
(0.372 g, 3.6 mmol) in methylene chloride (17 mL) was stirred overnight
at room temperature. The reaction was diluted with water, washed with
brine, dried (Na
2SO
4), and concentrated. The residue was purified by flash chromatography on silica gel (8%-10% MeOH:CH
2Cl
2) to give the title compound (0.515 g, 48%) as a pale yellow solid. MS (ES+) ink 592 [M+H]
+.
......................
WO2005107762
https://www.google.im/patents/WO2005107762A2
Example 1
cheme E:
ide
NaHCO
j, IPA 100 'C
1 , 1 -Dimethylethyl [( 1 S)-2-(4-bromophenyl)- 1 -(hydroxymethyl)ethyl]carbamate:
To
a solution of 4-bromo-N-{[(l ,1 -dimethylethyl)oxy] carbonyl }-L-
phenylalanine (72.6 mmol), in anhydrous diethyl ether (550 mL) at 0 °C
was added slowly lithium aluminum hydride, 95% (108.9 mmol). The
resulting solution was stiπed for an additional 2 h at 0 °C, The
reaction was then carefully quenched with a saturated aqueous solution
of sodium bicarbonate (73 mL) which stiπed at RT for half an hour.
Lithium aluminium salts crashed out of solution which were removed by
filtration. The filtrate was concentrated and vacuum pumped for 24 h to
afford the title product as a white solid (97%).
ESMS [M+H]
+: 331.2.
1,1 -Dimethylethyl {(lS)-2-(4-bromophenyl)-l-[(l,3-dioxo-l,3-dihydro-2H-isoindol-2- yl)methyl]ethyl}carbamate:
To a solution of 1 ,1 -dimethylethyl [(lS)-2-(4-bromophenyl)-l -
(hydroxymethyl)ethyl]carbamate
(70.6 mmol), tripheylphosphine (84.7 mmol), and phthalimide (84.7 mmol)
in anhydrous tetrahydrofuran (550 mL) at 0 °C was added dropwise
diisopropyl azodi carboxyl ate (84.7 mmol) over 10 minutes. The reaction
continued to stir allowing to wai to RT over 5h, The reaction was then
concentrated in vacuo and product was tritarated out of solution usingl
acetate (500 mL). The precipitate was filtered, washed with ethyl
acetate (3 x 100 mL), and dried to afford the title product as a white
solid (57%).
ESMS [M+H]
+: 460.4.
1 ,1 -Dimethylethyl {(15)-2-[4-(bromoacetyl)phenyl]-l -[(l,3-dioxo-l ,3-dihydro-2H-isoindol- 2-yl)methyl]ethyl}carbamate:

A
solution of 1,1 -dimethyl ethyl
{(lS)-2-(4-bromophenyl)-l-[(l,3-dioxo-l,3-
dihydro-2H-isoindol-2-yl)methyl]ethyl}carbamate (21.7 mmol),
1-ethoxyvinyltri-n-butylin (43.5 mmol), and
/ra/?s--dichlorobis(triphenylphospine)palladιum(II) (5 mol%) were stiπed
in anhydrous dioxane (300 mL) at 100 °C for 3h. The reaction was then
concentrated in vacuo and redissolved in a solution of tetrahydrofuran
and water (3:1, 400mL) and treated with N- bromosuccinimide (108.8 mmol)
and stined at RT for half an hour. The reaction solution was then
concentrated to dryness and redissolved in ethyl acetate (150 mL) and
precipate formed upon addition of hexanes (350 mL). The precipitate was
filtered and dried to afford the title product as yellow solid (71%).
ESMS [M+Η]+: 502.4. l,l-Dimethylethyl [(lS)-2-(l
,3-dioxo-l,3-dihydro-2H-isoindol-2-yl)-l-({4-[8-(l-
hydroxyethyl)imidazo[l,2-β]pyridin-2-yl]phenyl}methyl)ethyl]carbamate:
A mixture of l
!l-dimethylethyl{(lS)-2-{4-(biOinoacetyl)phenyl]-l-[(l,3-
dioxo-l ,3-dihydro-2H-isoindol-2-yl)methyl]ethyl}carbamate (1.90 g,
3.79 mmol), l-(2- amino-3-pyτidinyl)ethanol (0.523 g, 3.79 mmol), and
solid sodium bicarbonate (0.398 g, 4,72 mmol) in isopropanol (24 mL) was
refluxed for 3.0 h. and concentrated in vacuo. The residue was
dissolved in ethyl acetate, washed with water and saturated sodium
chloride, dried (Na
2S0
4), and concentrated to give the title compound (1.79 g, S7%) as a light pink solid. MS(ES+) m/e 541 [M+Η]
+.
3-Chloro-N-[(lS)-2-(l,3-dioxo-l
,3-dihydro-2H-isoindol-2-yl)-l-({4-[8-(l-
hydroxyethyl)imidazo[l,2-Λ]pyridin-2-yl]phenyl}methyl)ethyl]-4-[(l -
methylethyl)oxy]benzamide:
A
mixture of 1,1 -dimethylethyl
[(15)-2-(l,3-dioxo-l,3-dihydro-2H-isoindol-2-
yl)-l-({4-[8-(l-hydroxyethyl)imidazo[l,2-fl]pyridin-2-yl]phenyl}methyl)ethyl]carbamate
(1.79 g, 3.31 mmol) and 4M ΗC1 in 1,4-dioxane (20 mL, 80 mmol) was
stirred at room temperature for 45 minutes. The reaction was
concentrated to dryness ,redissolved in DMF (30 mL), and to this
solution was added N,N-diisopropylethylamine (2.14 g, 16,55 mmol) and
pentafluorophenyl 3-chloro-4 [(l-methylethyl)oxy]benzoate (1.36 g, 3.31
mmol). The mixture was stirred overnight at room temperature, diluted
with water, and extracted into ethyl acetate. The extracts were washed
with water, dried (Na SO ), and concentrated in vacuo to give the title
compound (2.10 g, 100%) as a tan solid. MS(ES+) m/e 637 [M+H]
+.
N-[(lS)-2-Amino-l-({4-[8-(l-hydroxyethyl)imidazo[l,2-α]p>tidin-2-
yl]phenyl}methyl)eth)'l]-3-chloro-4-[(l-methylethyl)oxy]benzamide:
A mixture of 3-chloro-N-[(lS)-2-(l,3-dioxo-l ,3-dihydro-2N-isoindol-2-yl)-l-
({4-[8-(l
-hydiOxyethyl)imidazo[l,2-β]pyridin-2-yl]phenyl}methyl)ethyl]-4-[(l-
methylethyl)oxy]benzamide (2.10 g, 3.30 mmol) and hydrazine monohydrate
(0.83 g, 16.5 mmol) in ethanol (30 mL) was heated at 57°C ovemight. The
reaction was cooled, diluted with ethanol, filtered, and concentrated to
give the title compound(1.67 g, 100%) as a pale yellow powder. MS(ES+)
m/e 507 [M+H]
+.
3-Chloro-N-[(15)-2-[(7VN-dimethylglycyl)amino]-l-({4-[8-(l-hydroxyethyl)imidazo[l
,2-
«]pyitdin-2-yl]phenyl}methyl)ethyl]-4-[(l-methylethyl)oxy]benzamide:
A
mixture ofN-[(lS)-2-amino-l-({4-[S-(l-hydroxyethyl)imidazo[l,2-
α]pyridin-2-yl]phenyl)methyl)ethyl]-3-chloro-4-[(l-methylethyl)oxy]benzamide
(0.912 g, 1 ,80 mmol), EDCI (0.69 g, 3,6 mmol),
NN-diisopropylethylamine (0.466 g, 3,6 mmol), and N,N-dimethylglycine
(0.372 g, 3.6 mmol) in methylene chloride (17 mL) was stirred overnight
at room temperature. The reaction was diluted with water, washed with
brine, dried (Νa
2S0 ), and concentrated. The residue was purified by flash chromatography on silica gel (8%-10% MeOH:CH
2Cl
2) to give the title compound ( 0.515 g, 48%) as a pale yellow solid. MS(ES+) m/e 592 [M+H]
+.
SEE
WO2008 / 138561
....................
Organic Process Research & Development (2010), 14(5), 1254-1263
Org. Process Res. Dev., 2010, 14 (5), pp 1254–1263
DOI: 10.1021/op100186c
http://pubs.acs.org/doi/abs/10.1021/op100186c

The discovery and development of an efficient manufacturing route to the CENP-E inhibitor 3-chloro-N-{(1S)-2-[(N,N-dimethylglycyl)amino]-1-[(4-{8-[(1S)-1-hydroxyethyl]imidazo[1,2-a]pyridin-2-yl}phenyl)methyl]ethyl}−4-[(1-methylethyl)oxy]benzamide
(GSK923295A) is described. The existing route to GSK923295A was
expensive, nonrobust, used nonideal reagents, and consistently struggled
to deliver the API needed for clinical studies. The new synthesis
commences from the readily available l-phenylalaninol,
which is smoothly converted through to GSK923295A using key
Friedel−Crafts acylation as well as selective acylation chemistries.
Downstream chemistry to GSK923295A is both high yielding and robust, and
the resulting process has been demonstrated first on the kilo scale and
subsequently in the pilot plant where 55 kg was successfully prepared.
The resulting process is simple, uses cheaper raw materials, is greener
in that it avoids using aluminum, tin, and bromination chemistries, and
obviates the need for chromatographic purification. Also discussed are
the route derived impurities, how they were unambiguously prepared to
confirm structure and processing amendments to control their formation,
and enhancements to the new process to facilitate future processing.
1H NMR (400 MHz, CD3OD) δH 1.34 (6H, d, J = 6.0, (CH3)2), 1.59 (3H, d, J = 7.0, CH3CH), 2.21 (6H, s, N(CH3)2), 2.87−3.01 (4H, m, CH2Ph and CH2N(CH3)2), 3.49 (2H, m, CH2NPhthal), 4.50 (1H, m, CHNH), 4.70 (1H, m, (CH3)2CHO)), 5.49 (1H, q, J = 7.0, CHOH), 6.88 (1H, t, J = 7.0, H-j), 7.08 (1H, d, J = 7.5, H-b), 7.33−7.37 (3H, m, H-k and H-d), 7.63 (1H, dd, J = 7.5 and 2.0, H-c), 7.78 (1H, s, H-a), 7.83 (2H, d, J = 7.0, H-e), 8.09 (1H, m, H-h), 8.27 (1H, d, J = 8.0, H-i);
13C NMR (100 MHz, CD3OD) δC
22.2, 24.1, 39.3, 43.8, 46.1, 53.0, 63.7, 66.2, 73.0, 110.4, 113.8,
115.3, 121.2, 124.5, 126.1, 127.5, 128.4, 128.5, 130.6, 130.7, 133.3,
136.0, 139.4, 145.1, 146.1, 157.6, 168.5 and 173.6;
HRMS (ESI+) m/z calculated for [M+H]+ C32H39N5O4Cl 592.2691, found 592.2684.
.........................
ACS Medicinal Chemistry Letters (2010), 1(1), 30-34
http://pubs.acs.org/doi/abs/10.1021/ml900018m
Inhibition
of mitotic kinesins represents a novel approach for the discovery of a
new generation of anti-mitotic cancer chemotherapeutics. We report here
the discovery of the first potent and selective inhibitor of
centromere-associated protein E (CENP-E) 3-chloro-N-{(1S)-2-[(N,N-dimethylglycyl)amino]-1-[(4-{8-[(1S)-1-hydroxyethyl]imidazo[1,2-a]pyridin-2-yl}phenyl)methyl]ethyl}-4-[(1-methylethyl)oxy]benzamide (GSK923295; 1), starting from a high-throughput screening hit, 3-chloro-4-isopropoxybenzoic acid 2. Compound 1 has demonstrated broad antitumor activity in vivo and is currently in human clinical trials.
SEE
WO-2015037460
https://patentscope.wipo.int/search/en/detail.jsf;jsessionid=F8D2DAAA427F9EBAB6B7CE67A7EE0772.wapp1nC?docId=WO2015037460&recNum=1&maxRec=&office=&prevFilter=&sortOption=&queryString=&tab=FullText
Method for producing optically active 3-(biphenyl-4-yl)-2-[(t-butoxycarbonyl)amino]propan-1-ol
Process
for preparing optically active
3-(biphenyl-4-yl)-2-[(t-butoxycarbonyl)amino]propan-1-ol, useful as an
intermediate in the synthesis of pharmaceuticals described in
WO2005107762 and WO2008138561 (such as GSK-923295 and tubulysin
derivatives respectively). Appears to be a new area of interest to the
assignee.
..............
WO2010118207
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2010118207&recNum=278&docAn=US2010030350&queryString=%28SYK%29%2520&maxRec=1655
1:
Mayes PA, Degenhardt YY, Wood A, Toporovskya Y, Diskin SJ, Haglund E,
Moy C, Wooster R, Maris JM. Mitogen-activated protein kinase (MEK/ERK)
inhibition sensitizes cancer cells to centromere-associated protein E
inhibition. Int J Cancer. 2013 Feb 1;132(3):E149-57. doi:
10.1002/ijc.27781. Epub 2012 Sep 28. PubMed PMID: 22948716.
2:
Chung V, Heath EI, Schelman WR, Johnson BM, Kirby LC, Lynch KM, Botbyl
JD, Lampkin TA, Holen KD. First-time-in-human study of GSK923295, a
novel antimitotic inhibitor of centromere-associated protein E (CENP-E),
in patients with refractory cancer. Cancer Chemother Pharmacol. 2012
Mar;69(3):733-41. doi: 10.1007/s00280-011-1756-z. Epub 2011 Oct 22.
PubMed PMID: 22020315.
3: Lock RB, Carol H, Morton CL, Keir ST,
Reynolds CP, Kang MH, Maris JM, Wozniak AW, Gorlick R, Kolb EA, Houghton
PJ, Smith MA. Initial testing of the CENP-E inhibitor GSK923295A by the
pediatric preclinical testing program. Pediatr Blood Cancer. 2012
Jun;58(6):916-23. doi: 10.1002/pbc.23176. Epub 2011 May 16. PubMed PMID:
21584937; PubMed Central PMCID: PMC3163687.
4: Balamuth NJ, Wood
A, Wang Q, Jagannathan J, Mayes P, Zhang Z, Chen Z, Rappaport E,
Courtright J, Pawel B, Weber B, Wooster R, Sekyere EO, Marshall GM,
Maris JM. Serial transcriptome analysis and cross-species integration
identifies centromere-associated protein E as a novel neuroblastoma
target. Cancer Res. 2010 Apr 1;70(7):2749-58. doi:
10.1158/0008-5472.CAN-09-3844. Epub 2010 Mar 16. PubMed PMID: 20233875;
PubMed Central PMCID: PMC2848992.
5: Wood KW, Lad L, Luo L, Qian
X, Knight SD, Nevins N, Brejc K, Sutton D, Gilmartin AG, Chua PR, Desai
R, Schauer SP, McNulty DE, Annan RS, Belmont LD, Garcia C, Lee Y,
Diamond MA, Faucette LF, Giardiniere M, Zhang S, Sun CM, Vidal JD,
Lichtsteiner S, Cornwell WD, Greshock JD, Wooster RF, Finer JT, Copeland
RA, Huang PS, Morgans DJ Jr, Dhanak D, Bergnes G, Sakowicz R, Jackson
JR. Antitumor activity of an allosteric inhibitor of
centromere-associated protein-E. Proc Natl Acad Sci U S A. 2010 Mar
30;107(13):5839-44. doi: 10.1073/pnas.0915068107. Epub 2010 Feb 18.
PubMed PMID: 20167803; PubMed Central PMCID: PMC2851928.









 COCK WILL TEACH YOU NMR
COCK WILL TEACH YOU NMR COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE

 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE amcrasto@gmail.com
amcrasto@gmail.com