
SAXAGLIPTIN
| Saxagliptin | |
| CAS No.: | 361442-04-8 |
|---|---|
| Synonyms: |
|
| Formula: | C18H25N3O2 |
| Exact Mass: | 315.19500 |
| Molecular Weight: | 315.41000 |
SMILES:
Saxagliptin, (1S,3S,5S)-2-(2S)-2-Amino-2-(3-hydroxyadamantan-1-yl)-acetyl)-2-azabicyclo[3.1.0]hexane-3-carbonitrile of the following chemical structure:
is a dipeptidyl peptidase IV (DPP4) inhibitor. Saxagliptin is marketed under the trade name ONGLYZA® by Bristol-Myers Squibb for the treatment of type 2 diabetes.
Saxagliptin and its hydrochloride and trifluoroacetic acid salts are disclosed in U.S. Pat. No. 6,395,767. In addition, U.S. Pat. No. 7,420,079 discloses Saxagliptin and its hydrochloride, trifluoroacetic acid and benzoate salts, as well as Saxagliptin monohydrate.
U.S. 2009/054303 and the corresponding WO 2008/131149 application disclose several crystalline forms of Saxagliptin and of Saxagliptin salts. The crystalline forms of Saxagliptin reported in that patent application are a monohydrate (denoted there as form H-1), a hemihydrate (denoted there as form H0.5-2), a dihydrate (denoted form H2-1) and an anhydrous form (denoted there as N-3).
WO 2005/117841 (the '841 application) describes the cyclization of Saxagliptin to form the therapeutically inactive cyclic amidine. The '841 application reports that such cyclization can occur both in solid state and solution state.
WO 2010/115974 discloses Forms: I-S, HT-S, IV-S, and HT-IV-S of Saxagliptin hydrochloride.

Org. Process Res. Dev., 2009, 13 (6), pp 1169–1176
DOI: 10.1021/op900226j

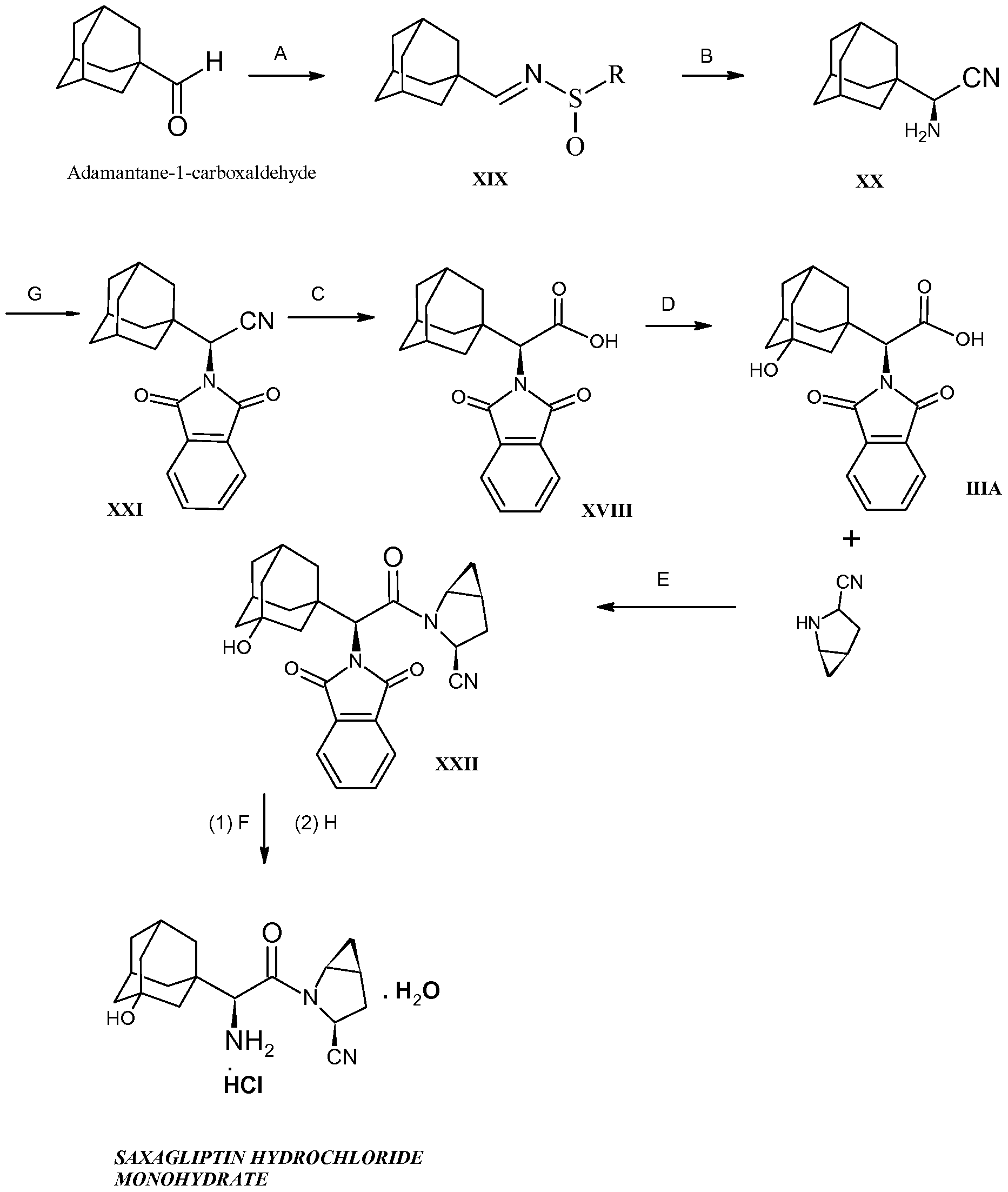
The commercial-scale synthesis of the DPP-IV inhibitor, saxagliptin (1), is described from the two unnatural amino acid derivatives 2 and 3. After the deprotection of 3, the core of 1 is formed by the amide coupling of amino acid 2 and methanoprolinamide 4. Subsequent dehydration of the primary amide and deprotection of the amine affords saxagliptin, 1. While acid salts of saxagliptin have proven to be stable in solution, synthesis of the desired free base monohydrate was challenging due to the thermodynamically favorable conversion of the free amine to the six-membered cyclic amidine 9. Significant process modifications were made late in development to enhance process robustness in preparation for the transition to commercial manufacturing. The impetus and rationale for those changes are explained herein.
Monohydrate 1 was isolated as a white solid (58.2 kg, 88%).
1 H NMR (400 MHz, CD2Cl2- d6) δ 5.25 (dd, J1 ) J2 ) 1.0 Hz, 1H), 4.93 (dd, J1 ) 10.6 Hz, J2 ) 2.3 Hz, 1H), 3.55-3.50 (m, 1H), 3,35 (s, 1H), 2.45 (ddd, J1 ) 16.1 Hz, J2 ) 10.9 Hz, J3 ) 5.6 Hz, 1H), 2.25 (dd, J1 ) 13.6 Hz, J2 ) 2.5 Hz, 1H), 2.18-2.10 (m, 2H), 1.83-1.42 (m, 15H), 1.40-1.27 (m, 3H) 1.0-0.87 (m, 2H)
13C NMR (100 MHz, CD2Cl2) δ 173.43, 120.15, 68.83, 60.90, 46.57, 45.51, 45.08, 45.01, 41.62, 38.15, 37.92, 37.35, 35.88, 30.98, 30.93, 30.80, 18.00, 13.69.
MS (FAB) m/z 316 [M + H]+
1H NMR PREDICT


.................. http://www.google.com/patents/WO2012162507A1?cl=en
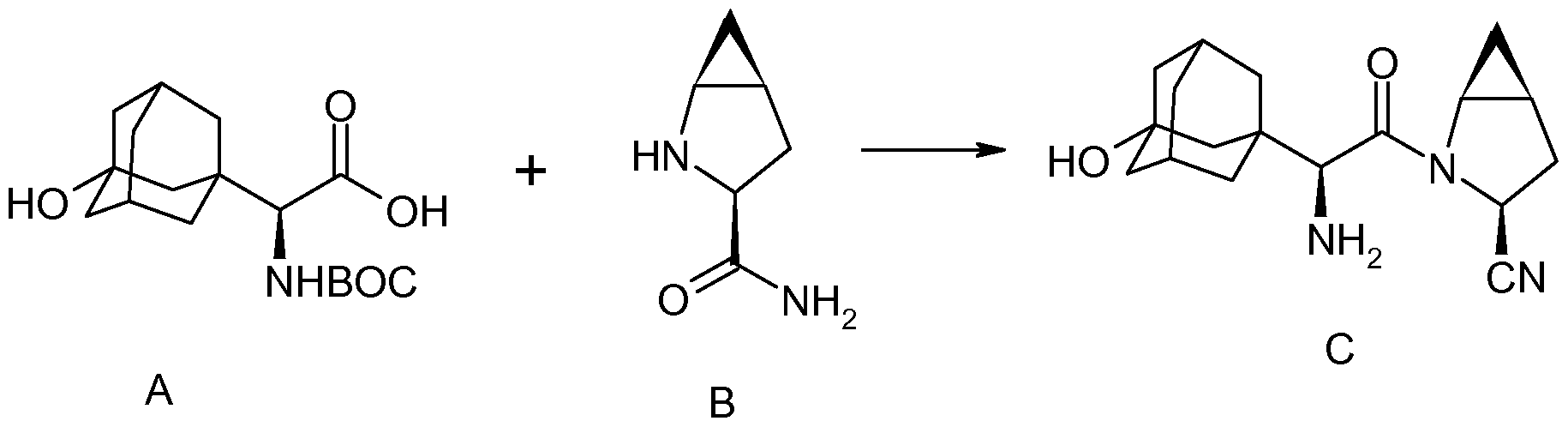
two amino acid derivatives (A) and (B), described in further detail hereinbelow, coupled in the presence of a coupling reagent. The amide coupling of (S)-a[[(l,l-dimethyleethoxy)carbonyl]amino]-3- hydroxytricyclo [3.3.1.1]decane-l-acetic acid (A) and (lS,3S,5S)-2-azabicyclo[3.1.0]hexane-3- carboxamide (B), subsequent dehydration of the primary amide and deprotection of the amine affords saxagliptin (C).
synthetic route is disclosed as follows:

Scheme-IV
.................. 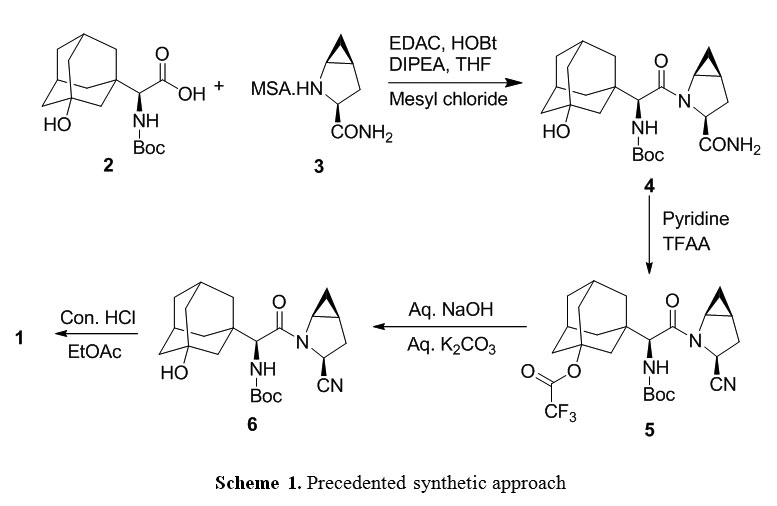 .................  .............   Savage, Scott A., et al., "Preparation of Saxagliptin, a Novel DPP-IV Inhibitor", Organic Process Research & Development, 2009, vol. 13, pp. 1169-1176. |
REFERENCES
| US6395767 | 16 Feb 2001 | 28 May 2002 | Bristol-Myers Squibb Company | Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV and method |
| US6995183 | 27 Jul 2004 | 7 Feb 2006 | Bristol Myers Squibb Company | Adamantylglycine-based inhibitors of dipeptidyl peptidase IV and methods |
| US7186846 | 28 Mar 2005 | 6 Mar 2007 | Bristol-Myers Squibb Company | Process for preparing a dipeptidyl peptidase IV inhibitor and intermediates employed therein |
| US7214702 | 23 May 2005 | 8 May 2007 | Bristol-Myers Squibb Company | Reacting the amide compound with phosphorus oxychloride in an organic solvent; treating the reaction mixture with water to form (1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxytricyclo[3.3.1.13,7]dec-1-yl)-1-oxoethyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile-hydrochloride |
| US7223573 | 2 May 2005 | 29 May 2007 | Bristol-Myers Squibb Company | Enzymatic ammonolysis process for the preparation of intermediates for DPP IV inhibitors |
| US7420079 | 18 Nov 2003 | 2 Sep 2008 | Bristol-Myers Squibb Company | Intermediates for making 1(alpha-amino-1-(cyclopropyl-fused pyrrolidinylcarbonyl)methyl)-3-hydroxyadamantanes, e.g., methyl 3-hydroxy-<a-oxotricyclo[3.3.1.13,7]decane-1-acetate |
| US7470810 | 11 Jan 2005 | 30 Dec 2008 | Bristol-Myers Squibb Company | Such as 1-dodecane-thiotrifluoroacetate; alkyl/arylthiol is treated with trifluoroacetic anhydride in presence of pyridine, solvent (dichloromethane), and dimethylaminopyridine (DMAP) as catalyst; for protection of amino acids |
| US7741082 | 12 Apr 2005 | 22 Jun 2010 | Bristol-Myers Squibb Company | Process for preparing dipeptidyl peptidase IV inhibitors and intermediates therefor |
| US7943656 | 18 Apr 2008 | 17 May 2011 | Bristol-Myers Squibb Company | Crystal forms of saxagliptin and processes for preparing same |
| US20060035954 | 8 Aug 2005 | 16 Feb 2006 | Sharma Padam N | Ammonolysis process for the preparation of intermediates for DPP IV inhibitors |
| WO2001068603A2 | 5 Mar 2001 | 20 Sep 2001 | Bristol Myers Squibb Co | Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl iv, processes for their preparation, and their use |
| WO2008131149A2 | 18 Apr 2008 | 30 Oct 2008 | Squibb Bristol Myers Co | Crystal forms of saxagliptin and processes for preparing same |
| WO2010115974A1 | 9 Apr 2010 | 14 Oct 2010 | Sandoz Ag | Crystal forms of saxagliptin |
| WO2011140328A1 | 5 May 2011 | 10 Nov 2011 | Teva Pharmaceutical Industries Ltd. | Saxagliptin intermediates, saxagliptin polymorphs, and processes for preparation thereof |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| US8748631 * | 24 May 2012 | 10 Jun 2014 | Apicore, Llc | Process for preparing saxagliptin and its novel intermediates useful in the synthesis thereof |
| US20130023671 * | 24 May 2012 | 24 Jan 2013 | Apicore, Llc | Process for preparing saxagliptin and its novel intermediates useful in the synthesis thereof |
- 1. Scott A. Savage, Gregory S. Jones, Sergei Kolotuchin, Shelly Ann Ramrattan, Truc Vu, and Rebert E. Waltermire (2009) Preparation of Saxagliptin, a Novel DPP-IV Inhibitor, Organic Process Research & Development., 13, 1169-1176.
- 2. Santosh K. Sing, Narendra Manne and Manojit Pal, (2008) Synthesis of (S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile: A key intermediate for dipeptidyl peptidase IV inhibitors. Beilstein Journal of Organic Chemistry, 4, No. 20.
- 3. U.S. Pat. No. (2010) 0274025 A1.
- 4. U.S. Pat. No. (2006) 0035954 A1.
- 5. U.S. Pat. No. (2005) 0090539 A1.
- 6. Organic letters. (2001) Vol. 3, No.5, Page: 759-762
- 7. Tetrahedron 59 (2003) 2953-2989
 COCK WILL TEACH YOU NMR
COCK WILL TEACH YOU NMR COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR

 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE

 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
 amcrasto@gmail.com
amcrasto@gmail.com
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.








VU-0483605, an isoindol derivative, has been found to be a mGluR1 positive allosteric modulator and probably be effective against schizophrenia. EC50: 356 and 390 nM for rat and human receptors, respectively. VU-0483605
ReplyDelete