
Currently there is a clinical trial that is recruiting patients from around the globe including sites across Australia. The trial is testing MM-398; a therapy that uses the latest in nanotechnology to deliver the chemotherapeutic agent irinotecan encased in a liposome to cancer patients.1 In particular this trial, named NAPOLI-1 (NAnoliPOsomaL Irinotecan) is recruiting patients with pancreatic cancer who have previously been treated with the chemotherapy agent gemcitabine unsuccessfully i.e. their disease has gone on to spread/progress despite this treatment.2,3
read all here
http://www.virtualmedicalcentre.com/news/new-therapy-for-pancreatic-cancer-phase-iii-clinical-trial-currently-recruiting-australian-patients/18708
irinotecan
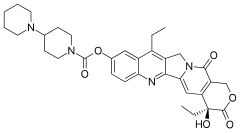
Irinotecan (Camptosar, Pfizer; Campto, Yakult Honsha) is a drug used for the treatment of cancer.
Irinotecan prevents DNA from unwinding by inhibition of topoisomerase 1. In chemical terms, it is a semisynthetic analogue of the natural alkaloid camptothecin.
Its main use is in colon cancer, in particular, in combination with other chemotherapy agents. This includes the regimen FOLFIRI, which consists of infusional 5-fluorouracil,leucovorin, and irinotecan.
Irinotecan received accelerated approval by the U.S. Food and Drug Administration (FDA) in 1996 and full approval in 1998. During development, it was known as CPT-11.
Irinotecan is activated by hydrolysis to SN-38, an inhibitor of topoisomerase I. This is then inactivated by glucuronidation by uridine diphosphate glucoronosyltransferase 1A1 (UGT1A1). The inhibition of topoisomerase I by the active metabolite SN-38 eventually leads to inhibition of both DNA replication and transcription.

Merrimack currently has six oncology therapeutics in clinical development, multiple product candidates in preclinical development and an active Systems Biology-driven discovery effort. MM-398
(Nanotherapeutic)
- Indication:
- Description:
- Target
- Pancreatic Cancer (2nd line, 2 indications), Colorectal Cancer, Glioma
- Nanotherapeutic
- Encapsulated irinotecan
MM-398 is a nanotherapeutic consisting of the chemotherapuetic irinotecan, encapsulated in a liposomal sphere. MM-398 is designed to rely on the natural blood flow of the tumor to direct the therapy directly to the site of the cancer and minimize exposure to non-target cells.
MM-398 in the Clinic
MM-398 is being evaluated in clinical trials for its ability to treat tumors resistant to chemotherapy across multiple types of cancers, including pancreatic, lung, colorectal and glioma. The FDA and the European Medicines Agency granted MM-398 orphan drug designation in 2011 for the treatment of patients with metastatic pancreatic cancer who have previously failed treatment with the chemotherapy drug gemcitabine. Our Phase 3 study, NAPOLI-1 (NAnoliPOsomaL Irinotecan), is currently underway.
posters
http://merrimackpharma.com/library/research/mm-398-preclinical-posters
posters
http://merrimackpharma.com/library/research/mm-398-preclinical-posters

Awesome,
ReplyDeleteThank you so much for sharing such an awesome blog...
body spa services for women in kukatpally