A condition called high cholesterol slowly creeps on you and you are unable to see its ill-effects until it becomes unbearable with spike to several complications such as diabetes, heart diseases and much more.
Other than just to pop up pills to keep the level of bad cholesterol or LDL under check, it is always a good choice to go for 5 must to have foods to keep the levels of high cholesterol under control. Thus, let’s go straight and see them. |
Tracks information on drugs on worldwide basis by Dr Anthony Melvin Crasto, helping millions with websites, 9 million hits on google, 2.5 lakh connections worldwide, P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
Friday, 26 July 2013
5 Must To Have Foods To Keep The Levels Of High Cholesterol Under Control
Why Vertex Earnings Prospects Are So Bright
Why Vertex Earnings Prospects Are So Bright
Motley Fool
With few drugs targeting cystic fibrosis and almost half of patients suffering from the particular mutation that the study targeted, Vertex could well have identified a blockbuster in the space. Moreover, with an ongoing phase 3 trial of another ...
http://www.fool.com/investing/general/2013/07/25/why-vertex-earnings-prospects-are-so-bright.aspx
Vertex Pharmaceuticals has a couple of approved drugs, including its Kalydeco cystic fibrosis drug and its Incivek treatment for hepatitis C. But it also has some promising prospects in the development stage. With the company having reported some positive study results during the quarter, Vertex saw its shares soar as more investors got aboard the biotech's bandwagon. Let's take an early look at what's been happening with Vertex Pharmaceuticals over the past quarter and what we're likely to see in its quarterly report
Motley Fool
With few drugs targeting cystic fibrosis and almost half of patients suffering from the particular mutation that the study targeted, Vertex could well have identified a blockbuster in the space. Moreover, with an ongoing phase 3 trial of another ...
http://www.fool.com/investing/general/2013/07/25/why-vertex-earnings-prospects-are-so-bright.aspx
Vertex Pharmaceuticals has a couple of approved drugs, including its Kalydeco cystic fibrosis drug and its Incivek treatment for hepatitis C. But it also has some promising prospects in the development stage. With the company having reported some positive study results during the quarter, Vertex saw its shares soar as more investors got aboard the biotech's bandwagon. Let's take an early look at what's been happening with Vertex Pharmaceuticals over the past quarter and what we're likely to see in its quarterly report
Thursday, 25 July 2013
Scripps Research Institute Scientists Find a Potential Cause of Parkinson’s Disease that Points to a New Therapeutic Strategy

For a high-resolution image see: http://www.scripps.edu/news/press/
images/reed_steve/reed_steve.jpg
LA JOLLA, CA – July 24, 2013 – Biologists at The Scripps Research Institute (TSRI) have made a significant discovery that could lead to a new therapeutic strategy for Parkinson’s disease.
The findings, recently published online ahead of print in the journal Molecular and Cell Biology, focus on an enzyme known as parkin, whose absence causes an early-onset form of Parkinson’s disease. Precisely how the loss of this enzyme leads to the deaths of neurons has been unclear. But the TSRI researchers showed that parkin’s loss sharply reduces the level of another protein that normally helps protect neurons from stress.
http://www.scripps.edu/news/press/2013/20130724reed.html
Wednesday, 24 July 2013
Isis Phase II drug APOIIIRx slashes triglycerides by 64%

Isis Pharmaceuticals is "very encouraged" by a second set of mid-stage data for its heart drugAPOIIIRx, which shows that it can substantially slash levels of dangerous fats in the blood.
In a 26-patient Phase II trial, patients with severely high levels of triglycerides taking Isis' drug alongside fibrates experienced 64% drop in triglycerides, and a 70% drop in apolipoprotein C-III (apoC-III), a component of 'bad' low-density lipoprotein
read all at
.http://www.pharmatimes.com/Article/13-07-23/Isis_PhII_drug_slashes_triglycerides_by_64.aspx

read all at
.http://www.pharmatimes.com/Article/13-07-23/Isis_PhII_drug_slashes_triglycerides_by_64.aspx

Labels:
anthony crasto,
APOIIIRx,
catalyst,
chemistry and application,
DRUGS,
herbs,
Isis,
ORGANIC SYNTHESIS,
PATENTS,
Phase II drug,
process,
triglycerides,
world drug tracker
Tuesday, 23 July 2013
New therapy for pancreatic cancer: Phase III clinical trial currently recruiting Australian patients

Currently there is a clinical trial that is recruiting patients from around the globe including sites across Australia. The trial is testing MM-398; a therapy that uses the latest in nanotechnology to deliver the chemotherapeutic agent irinotecan encased in a liposome to cancer patients.1 In particular this trial, named NAPOLI-1 (NAnoliPOsomaL Irinotecan) is recruiting patients with pancreatic cancer who have previously been treated with the chemotherapy agent gemcitabine unsuccessfully i.e. their disease has gone on to spread/progress despite this treatment.2,3
read all here
http://www.virtualmedicalcentre.com/news/new-therapy-for-pancreatic-cancer-phase-iii-clinical-trial-currently-recruiting-australian-patients/18708
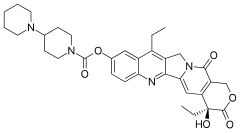
irinotecan
Irinotecan (Camptosar, Pfizer; Campto, Yakult Honsha) is a drug used for the treatment of cancer.
Irinotecan prevents DNA from unwinding by inhibition of topoisomerase 1. In chemical terms, it is a semisynthetic analogue of the natural alkaloid camptothecin.
Its main use is in colon cancer, in particular, in combination with other chemotherapy agents. This includes the regimen FOLFIRI, which consists of infusional 5-fluorouracil,leucovorin, and irinotecan.
Irinotecan received accelerated approval by the U.S. Food and Drug Administration (FDA) in 1996 and full approval in 1998. During development, it was known as CPT-11.
Irinotecan is activated by hydrolysis to SN-38, an inhibitor of topoisomerase I. This is then inactivated by glucuronidation by uridine diphosphate glucoronosyltransferase 1A1 (UGT1A1). The inhibition of topoisomerase I by the active metabolite SN-38 eventually leads to inhibition of both DNA replication and transcription.

Merrimack currently has six oncology therapeutics in clinical development, multiple product candidates in preclinical development and an active Systems Biology-driven discovery effort. MM-398
(Nanotherapeutic)
- Indication:
- Description:
- Target
- Pancreatic Cancer (2nd line, 2 indications), Colorectal Cancer, Glioma
- Nanotherapeutic
- Encapsulated irinotecan
MM-398 is a nanotherapeutic consisting of the chemotherapuetic irinotecan, encapsulated in a liposomal sphere. MM-398 is designed to rely on the natural blood flow of the tumor to direct the therapy directly to the site of the cancer and minimize exposure to non-target cells.
MM-398 in the Clinic
MM-398 is being evaluated in clinical trials for its ability to treat tumors resistant to chemotherapy across multiple types of cancers, including pancreatic, lung, colorectal and glioma. The FDA and the European Medicines Agency granted MM-398 orphan drug designation in 2011 for the treatment of patients with metastatic pancreatic cancer who have previously failed treatment with the chemotherapy drug gemcitabine. Our Phase 3 study, NAPOLI-1 (NAnoliPOsomaL Irinotecan), is currently underway.
posters
http://merrimackpharma.com/library/research/mm-398-preclinical-posters
posters
http://merrimackpharma.com/library/research/mm-398-preclinical-posters

Phase III prostate cancer trial for 'homing' injection shows improvements

Phase III prostate cancer trial for 'homing' injection shows improvements
A new treatment for advanced prostate cancer that homes-in on tumours to deliver a high-energy burst of radiation to cancer cells has shown significant benefits in a large scale clinical trial.
The trial of 921 patients showed that treatment with the radioactive Radium-223 gave men with late-stage prostate cancer an average extra of 15 weeks of life.http://www.pharmaceutical-technology.com/news/newsphase-iii-prostate-cancer-trial-for-homing-injection-shows-benefit?WT.mc_id=DN_News
Monday, 22 July 2013
Blood cancers – perifosine

An important target in cancer treatment is the serine/threonine specific protein kinase Akt, or protein kinase B. This kinase is involved in the pathway that prevents cell death, inhibiting apoptosis, and therefore inhibiting Akt may lead to cancer cell death.
One such compound is perifosine, discovered by Aeterna Zentaris and being developed by Keryx Biopharmaceuticals.1 Numerous clinical trials have been carried out in solid tumours, with varying success, and it failed a Phase III trial in colorectal cancer in 2012. However, it continues in trials for blood cancers such as lymphomas, where it shows promise.
In one Phase I/II trial, it was evaluated in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma who had previously received bortezomib therapy.2 A total of 84 patients, three-quarters of whom were refractory to bortezomib, and half to both bortezomib and dexamethasone, were given 50mg/day of perifosine, plus 1.3mg/m2 bortezomib, and 20mg dexamethasone added if progression occurred. The overall response rate was 43% in the 73 evaluable patients – 65% in the bortezomib-relapsed group and 32% in bortezomib-refractory patients. It was generally well tolerated, with manageable gastrointestinal events and fatigue. The median progression-free survival was 6.4 months, and a median overall survival of 25 months – 22.5 months in those refractory to bortezomib.

Perifosine
http://www.manufacturingchemist.com/technical/article_page/Blood_cancers__perifosine/88596#sthash.2bv5XjRe.dpuf
Subscribe to:
Posts (Atom)
