Regeneron and Bayer Report Positive Phase 3 Results for EYLEA® (aflibercept) Injection in Myopic Choroidal Neovascularization (mCNV)
TARRYTOWN, N.Y., June 06, 2013 /PRNewswire/ -- Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN) and Bayer HealthCare today announced positive top-line results for EYLEA® (aflibercept) Injection from the Phase 3 MYRROR study in myopic choroidal neovascularization (mCNV).
http://www.pharmalive.com/regeneron-bayer-report-positive-phase-3-results-for-eylea

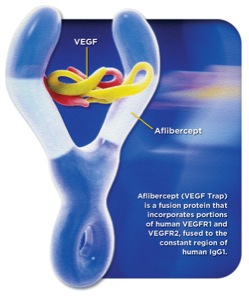
Aflibercept is a fusion protein approved in the United States for the treatment of wet macular degeneration and metastatic colorectal cancer.
It is an inhibitor of vascular endothelial growth factor. It is designed to bind to VEGF-A,VEGF-B, and placental growth factor (a.k.a PIGF, gene symbol PGF).[3]

No comments:
Post a Comment