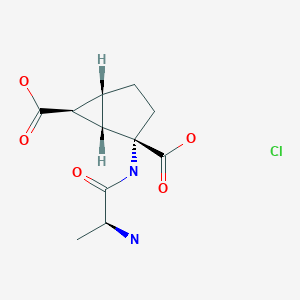
Talaglumetad hydrochloride
| |
| FORMULA | C11H16N2O5. HCl |
|---|---|
| EXACT MASS | 292.0826 |
| MOL WEIGHT | 292.7161 |
CAS: 441765-97-5
441765-98-6 (free base)
IUPAC Name: (1R,4S,5S,6S)-4-[[(2S)-2-aminopropanoyl]amino]bicyclo[3.1.0]hexane-4,6-dicarboxylic acid hydrochloride
Synonyms: Talaglumetad HCl, Talaglumetad hydrochloride, LY 544344 hydrochloride,
UNII-X30300EU7I, D09008, 441765-97-5,
Bicyclo(310)hexane-2,6-dicarboxylic acid, 2-(((2S)-2-amino-1-oxopropyl)amino)-, monohydrochloride, (1S,2S,5R,6S)-
(1S,2S,5R,6S)-2-(L-Alanylamino)bicyclo[3.1.0]hexane-2,6-dicarboxylic acid hydrochloride
(1S,2S,5R,6S)-2-[2(S)-Aminopropionamido]bicyclo[3.1.0]hexane-2,6-dicarboxylic acid hydrochloride
(1S,2S,5R,6S)-2-[2(S)-Aminopropionamido]bicyclo[3.1.0]hexane-2,6-dicarboxylic acid hydrochloride
Treatment of anxiety and stress disorders [metabotropic glutamate [mGlu] agonist]
see
Talaglumetad hydrochloride, a prodrug of the type II metabotropic glutamate receptor agonist eglumetad, reached phase III clinical studies for the treatment of anxiety at Lilly.
- In recent years, with the repeated cloning of glutamate receptor genes, it has become clear that there are surprisingly many subtypes of glutamate receptors. At present, glutamate receptors are broadly classified into two types: the “ionotropic type”, in which the receptor has an ion channel structure, and the “metabotropic type”, in which the receptor is coupled to G-proteins (Science, 258, 597-603, 1992). Ionotropic receptors are classified pharmacologically into three types: N-methyl-D-asparaginic acid (NMDA), α-amino-3-hydroxy-5-methyl isoxazole-4-propionate AMPA), and kynate (Science, 258, 597-603, 1992). Metabotropic receptors are classified into eight types, type 1 through type 8 (J. Neurosci., 13, 1372-1378, 1993; Neuropharmacol., 34, 1-26, 1995).
- The metabotropic glutamate receptors are classified pharmacologically into three groups. Of these, group 2 (mGluR2/mGluR3) bind with adenylcyclase, and inhibit the accumulation of the Forskolin stimulation of cyclic adenosine monophosphate (cAMP) (Trends Pharmacol. Sci., 14, 13 (1993)), which suggests that compounds that act on group 2 metabotropic glutamate receptors should be useful for the treatment or prevention of acute and chronic psychiatric and neurological disorders. As a substance that acts on group 2 metabotropic glutamate receptors, (+)-(1S,2S,5R,6S)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid has been disclosed in Japanese Unexamined Patent Publication, No. Hei 8-188561 [1996].
- Fluorine atoms tend to be strongly electron-attractive and to confer high fat solubility, and compounds into which fluorine atoms are introduced greatly change their physical properties. Thus introducing fluorine atoms might greatly affect the absorbability, metabolic stability, and pharmacological effects of a compound. But it is by no means easy to introduce fluorine atoms. In fact, Japanese Unexamined Patent Publication No. Hei 8-188561 [1996] does not even discuss the introduction of fluorine atoms into (+)-(1S,2S,5R,6S)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid.
………………………………………………………….
Process development of (1S,2S,5R,6S)-spiro[bicyclo[3.1.0]hexane-2′,5′-dioxo-2,4′-imidazolidine]-6-carboxylic acid, (R)-alpha-methylbenzenemethanamine salt (LSN344309)
Org Process Res Dev 2006, 10(1): 28
Org Process Res Dev 2006, 10(1): 28
LY544344 hydrochloride 6 is Talaglumetad

Process development and a pilot-plant process for the synthesis of 4 and its resolution to obtain (1S,2S,5R,6S)-spiro[bicyclo[3.1.0]hexane-2‘,5‘-dioxo-2,4‘-imidazolidine]-6-carboxylic acid, (R)-α-methylbenzenemethanamine salt (5) are described. Starting from the inexpensive raw 2-cyclopenten-1-one and sulfur ylide 1 the racemic bicyclo keto ester 2 was synthesized. Reaction of 2 with potassium cyanide and ammonium carbonate under Bücherer−Berg’s reaction conditions affords racemic 3 in 80% yield. Hydrolysis of 3 followed by the resolution with (R)-(+)-α-methylbenzylamine gave 4 in excellent yield and purity under optimized conditions. The improvement of the original discovery process to accommodate safety and environmental requirements for scale-up in manufacturing facilities is also discussed.
LY544344 hydrochloride 6 is a new chemical entity under investigation by Eli Lilly & Company as a potential treatment of neurological or psychiatric disorders related to the mammalian central nervous system (CNS)

Scheme 1. Original process for the synthesis of LSN344309 an intermediate of Talaglumetad
…………………………………………………….
Journal of Medicinal Chemistry (2005), 48(16), 5305-5320

…………………………………………………….
WO 2002055485
OR;

………………………………………………………….

………………………………………………
REFERENCES
New approaches in the development of orally bioavailable selective group 2 metabotropic glutamate receptor agonists
Drugs Fut 2002, 27(Suppl. A): Abst C39
Drugs Fut 2002, 27(Suppl. A): Abst C39
Utility of influx transporters to enhance oral bioavailability
241st ACS Natl Meet (March 27-30, Anaheim) 2011, Abst MEDI 163
241st ACS Natl Meet (March 27-30, Anaheim) 2011, Abst MEDI 163
The intestinal absorption of a prodrug of the mGlu2/3 receptor agonist LY354740 is mediated by PEPT1: In situ rat intestinal perfusion studies
J Pharm Sci 2010, 99(3): 1574
J Pharm Sci 2010, 99(3): 1574
Dipeptides as effective prodrugs of the unnatural amino acid (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740), a selective group II metabotropic glutamate receptor agonist
J Med Chem 2005, 48(16): 5305
J Med Chem 2005, 48(16): 5305
An efficient synthesis of LY544344.HCl: A prodrug of mGluR2 agonist LY354740
Tetrahedron Lett 2005, 46(43): 7299
Tetrahedron Lett 2005, 46(43): 7299
Pharmacodynamics of a novel anxiolytic (LY544344)
24th CINP Congr (June 20-24, Paris) 2004, Abst P02.269
24th CINP Congr (June 20-24, Paris) 2004, Abst P02.269
| WO2000004010A1* | Jul 14, 1999 | Jan 27, 2000 | Stephen Richard Baker | Bicyclohexane derivatives |
| EP0696577A1 * | Aug 11, 1995 | Feb 14, 1996 | Eli Lilly And Company | Synthetic excitatory amino acids |
| EP1052246A1 * | Jan 27, 1999 | Nov 15, 2000 | Taisho Pharmaceutical Co. Ltd | Fluorine-containing amino acid derivatives |
No comments:
Post a Comment