
LEDIPASVIR, GS 5885
CAS 1256388-51-8, PHASE 3
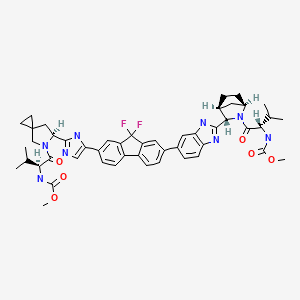
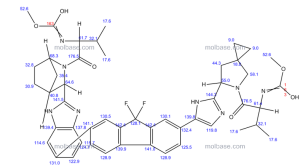
Methyl N-[(2S)-1-[(6S)-6-[5-[9,9-Difluoro-7-[2-[(1S,2S,4R)-3-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]-3-azabicyclo[2.2.1]heptan-2-yl]-3H-benzimidazol-5-yl]fluoren-2-yl]-1H-imidazol-2-yl]-5-azaspiro[2.4]heptan-5-yl]-3-methyl-1-oxobutan-2-yl]carbamate
GS-5885 had been in phase III clinical development at Gilead for the oral treatment of chronic genotype 1 hepatitis C virus (HCV) infection, however no recent developments have been reported on this research.
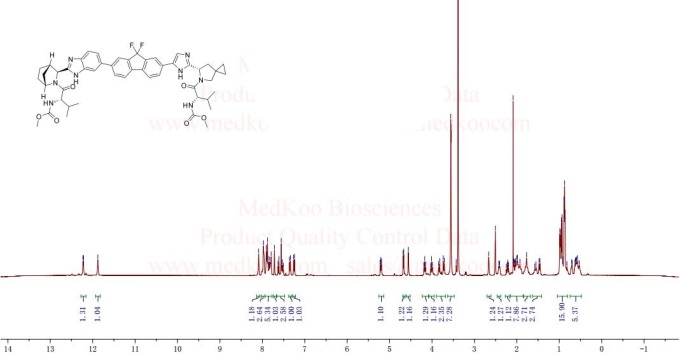
NMR LEDIPASVIR FROM NET
DMSOD6
Chronic Hepatitis C virus (HCV) infection is a global health problem with an estimated 170 million individuals infected worldwide. HCV infection is a major European public health challenge, with a prevalence of 0.4-3.5% in different EU member states. It is the most common single cause of liver transplantation in the Union. HCV is divided into six major genotypes and numerous subtypes, which are based on phylogenetic relationship. Genotype 1 is the most common genotype in Europe, comprising approximately 70 % of infections. Genotype 3 is second most common, followed by genotype 2. Genotype 4 is predominant in Egypt, the nation in the world with the highest documented HCV prevalence. Genotypes 5 and -6 are uncommon in Europe and the US, but are more common in South Africa and South-East Asia, respectively (Simmonds et al, Hepatology 2005). HCV genotype does not clearly impact the rate of disease progression. Treatment response, or the required drug pressure (number of drugs, treatment duration) needed to obtain maximal activity with presently approved regimens, differs between genotypes. The goal of antiviral therapy against HCV is to reach sustained virological response (SVR), which has traditionally been defined as the absence of quantifiable virus in plasma at least 24 weeks after the end of therapy (SVR24). However, most relapses occur within 4 weeks of treatment discontinuation, and a 98-99% concordance has been shown between absence of quantifiable virus 12 weeks after therapy, and SVR24 (Florian et al, AASLD 2011). Therefore the absence of measurable virus 12 weeks post end of treatment (SVR12) is presently considered accepted by European and US regulators as the primary endpoint in clinical trials. Though occasional late relapses occur, in general the durability of SVR has been demonstrated (e.g., Ng and Saab, Clin Gastroenterol Hepatol 2011). Of note, SVR4 (absence of quantifiable virus 4 weeks after treatment discontinuation) has an approximately 90% positive predictive value for SVR24 (Florian et al, AASLD 2011). Until the European Commission marketing authorisation of sofosbuvir in January 2014, all approved therapeutic regimens for the treatment of chronic HCV infection contained an interferon. For the treatment of genotype 1 infection, the addition of either one of the NS3/4A protease inhibitors telaprevir or boceprevir, authorised in 2011, was considered standard of care. For genotypes other than GT-1, there were no direct-acting antivirals (DAA) authorised, therefore dual therapy with pegIFN/RBV was the standard of care. Interferon-based therapies have limited efficacy in many patients and are associated with potentially serious side effects that are important in limiting real life effectiveness. These include a risk of hepatic decompensation and septicaemia in patients with advanced liver disease, as well as bone marrow suppression. Also, there are psychiatric side effects such as depression, which considerably limits eligibility to treatment in the target population (e.g., Bini et al, Am J Gastroenterol 2005). For these reasons, the development of highly effective interferon-free regimens for the treatment of hepatitis C targets addresses an important previously unmet medical need. SOF/LDV is a fixed-dose combination (FDC) tablet containing sofosbuvir (a previously approved NS5B polymerase inhibitor) and ledipasvir, a new NS5A-inhibitor. HCV NS5A is a multifunctional protein with key functions in HCV replication, virus assembly, and the modulation of cellular signaling pathways (e.g., Sheel and Rice, Nature Medicine, 2013). The FDC tablet contains 400 mg of SOF and 90 mg of LDV. SOF/LDV has the potential to be a simple and effective all-oral, once-daily treatment regimen for chronic HCV infection
REF
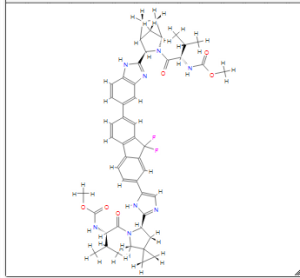
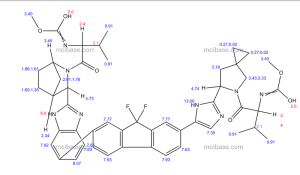
The chemical name of ledipasvir acetone solvate (LDV-AS) is methyl [(2S)-1-{(6S)-6-[5-(9,9-difluoro-7-{2-[(1R,3S,4S)-2-{(2S)-2-[(methoxycarbonyl)amino]-3-methylbut anoyl}-2-azabicyclo[2.2.1]hept-3-yl]-1H-benzimidazol-6-yl}-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-aza spiro[2.4]hept-5-yl}-3-methyl-1-oxobutan-2-yl]carbamate propan-2-one (1:1), also known as carbamic acid, N-[(1S)-1-[[(6S)-6-[5-[9,9-difluoro-7-[2-[(1R,3S,4S)-2-[(2S)-2-[(methoxycarbonyl)amino]-3-methyl- 1-oxobutyl]-2-azabicyclo[2.2.1]hept-3-yl]-1H-benzimidazol-6-yl]-9H-fluoren-2-yl]-1H-imidazol-2-yl]-5 -azaspiro[2.4]hept-5-yl]carbonyl]-2-methylpropyl]-, methyl ester, compd. with 2-propanone (1:1) or methyl {(1S)-1-[(1R,3S,4S)-3-{5-[9,9-difluoro-7-(2-{(6S)-5-[N- (methoxycarbonyl)- l-valyl]-5-azaspiro[2.4]hept-6-yl}-1Himidazol-4-yl)-9H-fluoren-2-yl]-1H-benzimidazol-2-yl}-2-azabicyc lo[2.2.1]heptane-2-carbonyl]-2-ethylpropyl}carbamate, compound with 2-propanone (1:1) and it has the following structure:
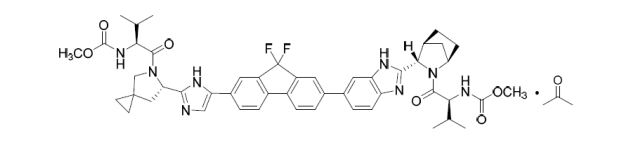
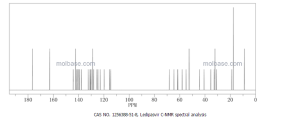
The structure of ledipasvir was unambiguously confirmed by 1 H, 13C and 19F NMR spectroscopy, UV spectroscopy, IR spectroscopy, high resolution mass spectrometry, elemental analysis and X-ray crystallography. LDV-AS is a white to tinted (off-white, tan, yellow, orange, or pink), slightly hygroscopic crystalline solid. It shows pH dependent solubility in aqueous media: it is slightly soluble in pH 2.3 buffer but practically insoluble in pH 4-7.5 buffers. It is freely soluble in ethanol and DMSO and slightly soluble in acetone. Ledipasvir is chiral and possesses 6 stereogenic centres and enantiomeric purity is controlled in starting material specifications. Three crystalline forms are known and ledipasvir acetone solvate is the designated commercial form. The first step for finished product manufacture involves the dissolution of ledipasvir in ethanol followed by spray-drying and thus precise control of morphology and particle size is not considered important. Ledipasvir is a chemical substance not previously authorised as a medicinal product in the European Union. Furthermore, it is not a salt, complex, derivative or isomer, (nor mixture of isomers), of a previously authorised substance. Whilst it contains some structural features in common with daclastavir, it is metabolically stable and the applicant presented data indicating that there are no common active metabolites. Therefore, the therapeutic moieties are not the same. Ledipasvir thus meets the definition of a New Active Substance according to the Notice to Applicants (NtA), Vol 2A, Chapter 1, Annex 3.
The mode of action of ledipasvir has not been directly established but indirect evidence is consistent with the compound targeting the NS5A molecule. In vitro resistance selection and cross-resistance studies, and the lack of HCV enzyme or kinase inhibition was taken to support the conclusion that ledipasvir targets NS5A as its mode of action. Ledipasvir has shown antiviral activity against HCV genotypes 1a and 1b with mean EC50 values of 0.031 and 0.004 nM, respectively. Antiviral activity determined as EC50 against genotypes 2 to 6 ranged from 0.15 to 530 nM. Ledipasvir showed no relevant antiviral activity at the highest concentration tested, or the highest concentration without cytotoxicity, against other virus such as bovine viral diarrhea virus (BVDV), RSV, HBV, HIV-1, HRV, influenza A and B, and a panel of flaviviruses (including West Nile virus, yellow fever virus, dengue virus, and banzai virus). Cytotoxicity of ledipasvir was characterised by CC50 of 4029 to >50000 nM using different cell lines (1b-Rluc-2, Huh-luc, 1a-HRlucp, Hep G2, SL3, Huh7, Hep-2, AD-38 and MT4 cells). Ledipasvir at 10 µM showed significant binding to 3 ion channels and 1 receptor in a radioligand binding assay screen against a panel of 68 mammalian ion channels and receptors. The IC50s of ledipasvir were 0.210 and 3.47 μM against sodium channel site 2 and calcium channel L-type (dihydropyridine), respectively. A 50% inhibition of androgen receptor was noted at 10 μM. Ledipasvir activity against 442 kinases was assessed using a quantitative polymerase chain reaction (qPCR)-based competition assay. Results showed weak competition for binding of 2 kinases, Bruton’s tyrosine kinase (BTK) and homeodomain-interacting protein kinase 1 (HIPK1) at 0.1 and 1 μM, respectively. Taking into account the high protein binding, >99.5%, of ledipasvir the large margin between unbound maximum clinical plasma levels (0.8 nM) and potential ion channel/receptor inhibition indicates limited clinical relevance.
Ledipasvir (formerly GS-5885) is a drug for the treatment of hepatitis C that was developed by Gilead Sciences.[1] After completingPhase III clinical trials, on February 10, 2014 Gilead filed for U.S. approval of a ledipasvir/sofosbuvir fixed-dose combination tablet for genotype 1 hepatitis C.[2][3] The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin.
Ledipasvir is an inhibitor of the hepatitis C virus NS5A protein.
Data presented at the 20th Conference on Retroviruses and Opportunistic Infections in March 2013 showed that a triple regimen of the nucleotide analog inhibitor sofosbuvir, ledipasvir, and ribavirin produced a 12-week post-treatment sustained virological response (SVR12) rate of 100% for both treatment-naive patients and prior non-responders with HCV genotype 1.[4][5] The sofosbuvir/ledipasvir coformulation is being tested with and without ribavirin. In February 2014 Gilead has filed for United StatesFood and Drug Administration (FDA) approval of ledipasvir/sofosbuvir oral treatment, without interferon and ribavirin.[6]
On October 10, 2014 the FDA approved the combination product ledipasvir 90 mg/sofosbuvir 400 mg called Harvoni.[7]
Similar to sofosbuvir, the cost of Harvoni has been a controversial topic. It costs $1,125 per pill in the US, translating to $63,000 for an 8-week treatment course, $94,500 for a 12-week treatment course, or $189,000 for a 24-week treatment course. Gilead justifies the cost by outweighing the benefit of curing hepatitis C over the cost of spending double on liver transplants or temporarily treating liver diseases. Gilead has provided a ledipasvir/sofosbuvir assistance program for eligible underserved or underinsured hepatitis C patients who cannot afford the costs of treatment. [10]

Hepatitis C is recognized as a chronic viral disease of the liver which is characterized by liver disease. Although drugs targeting the liver are in wide use and have shown
effectiveness, toxicity and other side effects have limited their usefulness. Inhibitors of hepatitis C virus (HCV) are useful to limit the establishment and progression of infection by HCV as well as in diagnostic assays for HCV.
The compound (l-{3-[6-(9,9-dif uoro-7-{2-[5-(2-methoxycarbonylamino-3-methyl- butyryl)-5-aza-spiro[2.4]hept-6-yl]-3H-imidazol-4-yl}-9H-fluoren-2-yl)-lH-benzoimidazol-2- yl]-2-aza-bicyclo[2.2.1]heptane-2-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester, also known as ledipasvir, designated herein as Compound I, is known to be an effective anti-HCV agent, as described for example in WO 2010/132601. A synthesis of compound I is disclosed in U.S. Patent No. 8,088,368


acetone solvate . 1H NMR (400 MHz, DMSO-^, δ): 12.29 (s, 0.1H), 12.19 (d, J=4.0 Hz, 1H), 12.14 (s, 0.2H), 11.85 (s, 1H), 8.10 (s, 0.1H), 8.08 (s, 1H), 8.01 (s, 0.1H), 7.963 (m, 1H), 7.955 (s, 1H), 7.89 (d, J=6.4 Hz, 1H), 7.87 (s, 1H), 7.83 (dd, J=8.4, 2.4 Hz, 1H), 7.79 (dd, J=7.2, 2.8 Hz, 1H), 7.78-7.90 (misc., 0.9H), 7.70 (s, 1H), 7.61 (d, J=8.4 Hz, 1H), 7.55 (s, 1H), 7.51 (dd, J=8.8, 1.6 Hz, 1H), 7.44 (m, 0.1H), 7.31 (d, J=8.4 Hz, 1H), 7.21 (d, J=8.4 Hz, 1H), 6.91 (d, J=8.0 Hz, 0.2H), 6.77 (m, 0.2H), 5.34 (d, J=7.6 Hz, 0.1H), 5.20 (dd, J=8.0, 5.2 Hz, 1H), 5.18 (m, 0.1H), 4.88 (s, 0.1H), 4.67 (d, J=6.4 Hz, 1H), 4.55 (s, 1H), 4.17 (dd, J=8.0, 8.0 Hz, 1H), 4.10 (m, 0.2H), 4.01 (dd, J=8.4, 8.0 Hz, 1H), 3.97 (m, 0.1H), 3.82 (d, J=9.6 Hz, 1H), 3.77 (s, 0.2H), 3.71 (d, J=9.6 Hz, 1H), 3.554 (s, 3H), 3.548 (s, 3H), 3.43 (s, 0.4H), 3.20 (d, J=7.6 Hz, 0.3H), 2.77 (s, 0.1H), 2.66 (s, 1H), 2.41 (d, J=8.8 Hz, 1H), 2.22 (dd, J=12.4, 8.0 Hz, 1H), 2.13 (m, 0.4H), 2.08 (s, 6H), 2.05 (dd, J=13.2, 5.2 Hz, 1H), 1.99 (m, 2H), 1.92 (m, 1H), 1.77 (m, 2H), 1.61 (m, 0.3H), 1.56 (m, 1H), 1.46 (d, J=9.2 Hz, 1H), 1.33 (d, J=10.0 Hz, 0.1H), 0.97 (dd, J=6.4, 2.0 Hz, 3H), 0.93 (d, J=6.8 Hz, 3H), 0.88 (d, J=6.4 Hz, 3H), 0.87 (d, J=6.4 Hz, 3H), 0.80-1.05 (misc., 2H), 0.70 (m, 1H), 0.59 (m, 2H), 0.54 (m, 1H), 0.33 (m, 0.1H). HRMS-ESI+: [M + H]+ calcd for C49H5506N8F2, 889.4207; found, 889.4205.
https://www.google.co.in/patents/WO2013184698A1
..................................................................................................
SYN


https://www.google.co.in/patents/WO2013184702A1



https://www.google.co.in/patents/WO2013184702A1


..........................................................................
INTERMEDIATES
.......................................
 ..............................................................
..............................................................PAPENT https://www.google.co.in/patents/US8088368 Example ED Preparation of Intermediate 5-Aza-spiro[2.4]heptane-5,6-dicarboxylic acid 5-benzyl ester 6-methyl ester
 4-Methylene-pyrrolidine-1,2-dicarboxylic acid 1-benzyl ester 2-methyl ester
4-Methylene-pyrrolidine-1,2-dicarboxylic acid 1-benzyl ester 2-methyl ester
4-Methylene-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester (10.0 g, 44 mmol) was dissolved in MeOH (75 mL) at room temperature and HCl (4M in dioxane, 75 mL) was added. Stirring at room temperature was continued for 4 hours. All volatiles were removed in vacuo and a beige solid was obtained.
The crude material was suspended in DCM (100 mL) and N-Methyl morpholine (13.3 g, 132 mmol) was added. The mixture was cooled to 0° C. and benzyl chloroformate (8.26 g, 48.4 mmol) was added while stirring. After 30 minutes, the reaction was warmed to room temperature and the solution was washed with water and aqueous HCl (1M). The solution was dried over sodium sulfate. Filtration and evaporation of solvents gave crude product, which was purified by silica gel chromatography (eluent: EtOAc/hexanes) to yield the product (10.2 g). LCMS-ESI+: calc'd for C15H17NO4: 275.3 (M+). Found: 276.4 (M+H+).
5-aza-spiro[2.4]heptanes-5,6-dicarboxylic acid benzyl ester: An oven-dried 3-neck round bottom flask was equipped with a nitrogen inlet adaptor and a 250 mL addition funnel. The third neck was sealed with a septum. The flask was charged with a stir bar, dichlorormethane (120 mL) and diethyl zinc (1.0 M in hexane, 118 mL, 118 mmol) then cooled to 0° C. in an ice bath. The addition funnel was charged with dichloromethane (40 mL) and trifluoroacetic acid (9.1 mL, 118 mmol). After the diethyl zinc solution had cooled to 0° C. (about 25 minutes), the trifluoroacetic acid solution was added dropwise over 20 minutes to the stirred reaction mixture. After stirring for another 20 minutes at 0° C., diiodomethane (9.5 mL, 118 mmol) was added slowly over 4 minutes. After another 20 minutes, 4-methylene-pyrrolidine-1,2-dicarboxylic acid 1-benzyl ester 2-methyl ester (8.10 g, 29.4 mmol) was added in 30 mL dichloromethane by cannula. The flask containing 4-methylene-pyrrolidine-1,2-dicarboxylic acid 1-benzyl ester 2-methyl ester was then rinsed with another 10 mL dichloromethane and this solution was also transferred to the reaction mixture by cannula. The reaction mixture was allowed to warm to RT and stirred for 110 hours (about 5 days) after which the reagents were quenched with saturated aqueous ammonium chloride (˜150 mL). The contents of the flask were slowly poured into a 2 L sep funnel containing saturated aqueous sodium bicarbonate (˜800 mL). The aqueous phase was extracted three times with 300 mL ethyl acetate. The combined organics were dried over magnesium sulfate and concentrated to provide the crude material. The crude material was dissolved in 3:1:1 THF/water/acetone (165 mL) then treated with N-methylmorpholine-N-oxide (3.45 g, 29.4 mmol) and osmium tetroxide (4 wt % in water, 5 mL, 0.818 mmol). After stirring at RT for 7 h, the reagents were quenched with 1 M aqueous sodium thiosulfate (˜100 mL). The contents of the flask were then poured into a 1 L sep funnel containing water (˜300 mL). The aqueous phase was extracted three times with 300 mL dichloromethane. The combined organics were dried over magnesium sulfate and concentrated. The crude residue was purified by silica column chromatography (5% to 45% EtOAc/hexane) to provide 5-aza-spiro[2.4]heptane-5,6-dicarboxylic acid 5-benzyl ester 6-methyl ester as a clear oil (5.54 g, 19.15 mmol, 65%) as a clear oil. 1H NMR (CDCl3) δ 7.36-7.29 (m, 5H), 5.21-5.04 (m, 2H), 4.56-4.47 (m, 1H), 3.75 (s, 1.5H), 3.60 (m, 1.5H), 03.51-3.37 (m, 2H), 2.32-2.25 (m, 1H), 1.87-1.80 (m, 1H), 0.64-0.51 (m, 4H).
5-Aza-spiro[2.4]heptane-5,6-dicarboxylic acid 5-benzyl ester
5-Aza-spiro[2.4]heptane-5,6-dicarboxylic acid 5-benzyl ester 6-methyl ester (244 mg, 0.840 mmol) was dissolved in THF (2.0 mL)/MeOH (1.5 mL) An aqueous solution of LiOH (35.5 mg, 0.84 mmol) was added and stirring at room temperature was continued. After 3 hours, the reaction was neutralized with aqueous HCl (1M) and the organic solvents were removed in vacuo. The crude mixture was diluted with water and EtOAc and the organic layer was collected. All volatiles were removed in vacuo and the crude acid was used without further purification. LCMS-ESI+: calc'd for C15H17NO4: 275.3 (M+). Found: 276.3 (M+H+).
Example ED′
2,7-Dibromo-fluoren-9-one (4.0 g, 11.8 mmol) was suspended in deoxofluor (12 mL) at room temperature and EtOH (4 drops) was added. The stirred suspension was heated at T=90° C. for 24 hours (CAUTION: Use of deoxofluor at elevated temperatures, as described above, is strongly discouraged as rapid and violent exotherms may occur). The reaction was cooled to room temperature and poured onto ice containing sodium bicarbonate. A solid formed and was collected via filtration. The crude material was taken into EtOAc and was washed with aqueous HCl (1M) and brine. The solution was dried over sodium sulfate. Filtration and evaporation of solvents gave crude product, which was purified by silica gel chromatography (eluent: EtOAc/hexanes) to yield the product 2,7-Dibromo-9,9-difluoro-9H-fluorene (3.2 g). 19F-NMR: 282 MHz, (dmso-d6) δ: −111.6 ppm.
Before using the material in the next step, it was exposed as a solution in EtOAc to charcoal.
5-Aza-spiro[2.4]heptane-5,6-dicarboxylic acid 5-benzyl ester 6-[2-(7-bromo-9,9-difluoro-9H-fluoren-2-yl)-2-oxo-ethyl]ester
2,7-Dibromo-9,9-difluoro-9H-fluorene (372 mg, 1.04 mmol), Pd(PPh3)4 (30.0 mg, 0.026 mmol), PdCl2(PPh3)2 (18.2 mg, 0.026 mmol), As(PPh3)3 (5.0 mg) were dissolved in dioxane (10 mL) under an argon atmosphere. Ethoxyvinyl-tributyl tin (376.4 mg, 1.04 mmol) was added. The mixture was heated for 140 minutes at 85° C. (oil bath). The reaction was cooled to room temperature. N-bromo succinimide (177 mg, 1.0 mmol) was added followed by water (2 mL). The reaction was stirred at room temperature for 3 hours, after which the majority of the dioxane was removed in vacuo. The crude reaction mixture was diluted with EtOAc and was washed with water. All volatiles were removed in vacuo. Toluene was added and all volatiles were removed in vacuo for a second time. The crude material was dissolved in DMF/MeCN (2 mL, 1:1) at room temperature. A solution of N-Cbz-4-cyclopropyl (L) Proline (0.84 mmol) and DIEA (268 mg, 2.08 mmol) in MeCN (2 mL) was added and stirring at room temperature was continued. After 14 hours, most of the MeCN was removed in vacuo and the crude reaction mixture was diluted with EtOAc. The mixture was washed with aqueous HCl (1M), aqueous LiCl solution (5%), brine, and was dried over sodium sulfate. Filtration and evaporation of solvents gave the crude reaction product, which was purified via silica gel chromatography (eluent: EtOAc/hexanes) to yield the product 5-Aza-spiro[2.4]heptane-5,6-dicarboxylic acid 5-benzyl ester 6-[2-(7-bromo-9,9-difluoro-9H-fluoren-2-yl)-2-oxo-ethyl]ester (176 mg). LCMS-ESI+: calc'd for C30H24BrF2NO5: 596.4 (M+). Found: 595.2/597.2 (M+H+).
6-[5-(7-Bromo-9,9-difluoro-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-aza-spiro[2.4]heptane-5-carboxylic acid benzyl ester
5-Aza-spiro[2.4]heptane-5,6-dicarboxylic acid 5-benzyl ester 6-[2-(7-bromo-9,9-difluoro-9H-fluoren-2-yl)-2-oxo-ethyl]ester (172 mg, 0.293 mmol) was dissolved in m-xylenes (6.0 mL). Ammonium acetate (226 mg, 2.93 mmol) was added and the reaction was stirred at 140° C. for 60 minutes under microwave conditions. The reaction was cooled to room temperature and all volatiles were removed in vacuo. The crude material was purified via silica gel chromatography (eluent: EtOAc/hexanes) to yield the product 6-[5-(7-Bromo-9,9-difluoro-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-aza-spiro[2.4]heptane-5-carboxylic acid benzyl ester (80.3 mg). LCMS-ESL': calc'd for C30H24BrF2N3O2: 576.4 (M+). Found: 575.2/577.2 (M+H+).
(1-{6-[5-(7-Bromo-9,9-difluoro-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-aza-spiro[2.4]heptane-5-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester
6-[5-(7-Bromo-9,9-difluoro-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-aza-spiro[2.4]heptane-5-carboxylic acid benzyl ester (800 mg, 1.38 mmol) was dissolved in DCM (15 mL) and HBr in AcOH (37%, 2 mL) was added and stirring at room temperature was continued. After 180 minutes, the suspension was diluted with hexanes and the solid was collected via filtration and was washed with hexanes and subjected to vacuum. The crude material was used in the next step without further purification. The crude material was dissolved in DMF (4.0 mL) and DIEA (356 mg, 2.76 mmol) was added. A solution of 2-(L)-Methoxycarbonylamino-3-methyl-butyric acid (242 mg, 1.38 mmol), HATU (524 mg, 1.38 mmol) and DIEA (178 mg, 1.38 mmol) in DMF (1 mL) was added. The reaction was stirred at room temperature. After 50 minutes, the reaction was diluted with EtOAc and was washed with aqueous bicarbonate solution, aqueous LiCl solution (5%), brine, and was dried over sodium sulfate. Filtration and removal of solvents in vacuo gave the crude material, which was purified by silica gel chromatography (eluent: EtOAc/hexanes) to yield the slightly impure product (1-{6-[5-(7-Bromo-9,9-difluoro-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-aza-spiro[2.4]heptane-5-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester (878 mg). LCMS-ESI+: calc'd for C29H29BrF2N4O3: 599.5 (M+); Found: 598.5/600.5 (M+H+).
3-[6-(9,9-Difluoro-7-{2-[5-(2-methoxycarbonylamino-3-methyl-butyryl)-5-aza-spiro[2.4]hept-6-yl]-3H-imidazol-4-yl}-9H-fluoren-2-yl)-1H-benzoimidazol-2-yl]-2-aza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester
(1-{6-[5-(7-Bromo-9,9-difluoro-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-aza-spiro[2.4]heptane-5-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester (840 mg, 1.4 mmol), 3-[6-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-benzoimidazol-2-yl]-2-aza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester (615 mg, 1.4 mmol), Pd(PPh3)4 (161 mg, 0.14 mmol), K2CO3 (579 mg, 4.2 mmol), were dissolved in DME (15 mL)/water (3 mL) under an argon atmosphere. The mixture was heated for 120 minutes at 85-90° C. (oil bath). After 120 minutes additional boronate ester (61 mg, 0.14 mmol) was added and heating was continued. After 3 hours, the reaction was cooled to room temperature. Most of the DME was removed in vacuo and the crude reaction mixture was diluted with EtOAc. The mixture was washed with brine and was dried over sodium sulfate. Filtration and evaporation of solvents gave the crude reaction product, which was purified via silica gel chromatography (eluent: EtOAc/hexanes) to yield the product 3-[6-(9,9-Difluoro-7-{2-[5-(2-methoxycarbonylamino-3-methyl-butyryl)-5-aza-spiro[2.4]hept-6-yl]-3H-imidazol-4-yl}-9H-fluoren-2-yl)-1H-benzoimidazol-2-yl]-2-aza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester (878 mg). LCMS-ESI+: calc'd for C47H51F2N7O5: 831.9 (M+). Found: 832.7 (M+H+).
(1-{3-[6-(9,9-Difluoro-7-{2-[5-(2-methoxycarbonylamino-3-methyl-butyryl)-5-aza-spiro[2.4]hept-6-yl]-3H-imidazol-4-yl}-9H-fluoren-2-yl)-1H-benzoimidazol-2-yl]-2-aza-bicyclo[2.2.1]heptane-2-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester
3-[6-(9,9-Difluoro-7-{2-[5-(2-methoxycarbonylamino-3-methyl-butyryl)-5-aza-spiro[2.4]hept-6-yl]-3H-imidazol-4-yl}-9H-fluoren-2-yl)-1H-benzoimidazol-2-yl]-2-aza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester (115 mg, 0.138 mmol) was dissolved in DCM (2 mL) and HCl in dioxane (4M, 2 mL) was added and stirring at room temperature was continued. After 20 minutes, all volatiles were removed in vacuo. The crude material was used in the next step without further purification. The crude material was dissolved in DMF (1.5 mL) and DIEA (53.4 mg, 0.414 mmol) was added. A solution of 2-(L) Methoxycarbonylamino-3-methyl-butyric acid (24.2 mg, 0.138 mmol), HATU (52.4 mg, 0.138 mmol) and DIEA (17.8 mg, 0.138 mmol) in DMF (1 mL) was added. The reaction was stirred at room temperature. After 20 minutes, the reaction was diluted with EtOAc and was washed with aqueous bicarbonate solution, aqueous LiCl solution (5%), brine, and was dried over sodium sulfate. Filtration and removal of solvents in vacuo gave the crude material, which was purified by RP-HPLC (eluent: water/MeCN w/0.1% TFA) to yield the product (1-{3-[6-(9,9-Difluoro-7-{2-[5-(2-methoxycarbonylamino-3-methyl-butyryl)-5-aza-spiro[2.4]hept-6-yl]-3H-imidazol-4-yl}-9H-fluoren-2-yl)-1H-benzoimidazol-2-yl]-2-aza-bicyclo[2.2.1]heptane-2-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester (76 mg). LCMS-ESI+: calc'd for C49H54F2N8O6: 888.9 (M+). Found: 890.0 (M+H+).
1H-NMR: 300 MHz, (dmso-d6) δ: 8.20-7.99 (m, 8H), 7.73 (s, 2H), 7.37-7.27 (m, 2H), 5.25 (dd, J=7.2 Hz, 1H), 4.78 (s, 1H) 4.54 (s, 1H), 4.16 (m, 1H), 4.02 (m, 1H), 3.87 (m, 1H), 3.74 (m, 1H), 3.55 (s, 3H), 3.53 (s, 3H), 2.75 (m, 1H), 2.25 (m, 2H), 2.09-2.04 (m, 2H), 1.88-1.79 (m, 2H), 1.54 (m, 1H), 0.94-0.77 (m, 15H) 0.63 (m, 4H) ppm. 19F-NMR: 282 MHz, (dmso-d6) δ: −109.1 ppm [−74.8 ppm TFA]
https://www.google.co.in/patents/US8088368
 2-(5-{9,9-Difluoro-7-[2-(2-Boc-2-aza-bicyclo[2.2.1]hept-3-yl)-3H-benzoimidazol-5-yl]-9H-fluoren-2-yl}-1H-imidazol-2-yl)-pyrrolidine-1-carboxylic acid tert-butyl ester: A mixture of 2-[5-(7-Bromo-9,9-difluoro-9H-fluoren-2-yl)-1H-imidazol-2-yl]-pyrrolidine-1-carboxylic acid tert-butyl ester (324 mg, 0.627 mmol), 3-[6-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-benzoimidazol-2-yl]-2-aza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester (1.1 eq., 304 mg), [1,1′ bis(diphenylphosphino)ferrocene]dichloropalladium(II)(3%, 15 mg), tetrakis(triphenylphosphine)palladium (3%, 22 mg) and potassium carbonate (3.3 eq., 285 mg) in 10 mL DME and 3 mL water was heated to 90° C. under Argon for 3 hours. The reaction mixture was cooled and diluted with ethyl acetate and washed with saturated sodium bicarbonate solution. The organic layer was dried (MgSO4), concentrated and purified by flash column chromatography (silica gel, 20 to 100% ethyl acetate/hexane) to give 2-(5-{9,9-Difluoro-7-[2-(2-Boc-2-aza-bicyclo[2.2.1]hept-3-yl)-3H-benzoimidazol-5-yl]-9H-fluoren-2-yl}-1H-imidazol-2-yl)-pyrrolidine-1-carboxylic acid tert-butyl ester (361 mg, yield 77%). LCMS-ESI−: calc'd for C43H46F2N6O4: 748.86. Found: 749.2 (M+H+).
2-(5-{9,9-Difluoro-7-[2-(2-Boc-2-aza-bicyclo[2.2.1]hept-3-yl)-3H-benzoimidazol-5-yl]-9H-fluoren-2-yl}-1H-imidazol-2-yl)-pyrrolidine-1-carboxylic acid tert-butyl ester: A mixture of 2-[5-(7-Bromo-9,9-difluoro-9H-fluoren-2-yl)-1H-imidazol-2-yl]-pyrrolidine-1-carboxylic acid tert-butyl ester (324 mg, 0.627 mmol), 3-[6-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-benzoimidazol-2-yl]-2-aza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester (1.1 eq., 304 mg), [1,1′ bis(diphenylphosphino)ferrocene]dichloropalladium(II)(3%, 15 mg), tetrakis(triphenylphosphine)palladium (3%, 22 mg) and potassium carbonate (3.3 eq., 285 mg) in 10 mL DME and 3 mL water was heated to 90° C. under Argon for 3 hours. The reaction mixture was cooled and diluted with ethyl acetate and washed with saturated sodium bicarbonate solution. The organic layer was dried (MgSO4), concentrated and purified by flash column chromatography (silica gel, 20 to 100% ethyl acetate/hexane) to give 2-(5-{9,9-Difluoro-7-[2-(2-Boc-2-aza-bicyclo[2.2.1]hept-3-yl)-3H-benzoimidazol-5-yl]-9H-fluoren-2-yl}-1H-imidazol-2-yl)-pyrrolidine-1-carboxylic acid tert-butyl ester (361 mg, yield 77%). LCMS-ESI−: calc'd for C43H46F2N6O4: 748.86. Found: 749.2 (M+H+).
(1-{2-[5-(9,9-Difluoro-7-{2-[2-(2-methoxycarbonylamino-3-methyl-butyryl)-2-aza-bicyclo[2.2.1]hept-3-yl]-3H-benzoimidazol-5-yl}-9H-fluoren-2-yl)-1H-imidazol-2-yl]-pyrrolidine-1-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester (Example DK): 4N HCl in dioxane (2 mL) was added to 2-(5-{9,9-Difluoro-7-[2-(2-Boc-2-aza-bicyclo[2.2.1]hept-3-yl)-3H-benzoimidazol-5-yl]-9H-fluoren-2-yl}-1H-imidazol-2-yl)-pyrrolidine-1-carboxylic acid tert-butyl ester (361 mg, 0.482 mmol) and the reaction mixture was stirred at room temperature for 4 hours. The reaction mixture was concentrated and dried overnight under vacuum. The residue was dissolved in DMF (5 mL) and to this solution was added 2-Methoxycarbonylamino-3-methyl-butyric acid (2 eq., 169 mg), diisopropyl ethylamine (6 eq., 0.5 mL), followed by HATU (2 eq., 367 mg). Reaction mixture was stirred at 0° C. for 30 minutes. The reaction mixture was dissolved in ethyl acetate and washed with saturated sodium bicarbonate solution. The organic layer was dried (MgSO4), concentrated and purified by flash column chromatography (silica gel, 0 to 20% MeOH/ethyl acetate), followed by preparative reverse phase HPLC (GEMINI, 5 to 100% ACN/H2O+0.1% TFA). Product was lyophilized to give (1-{2-[5-(9,9-Difluoro-7-{2-[2-(2-methoxycarbonylamino-3-methyl-butyryl)-2-aza-bicyclo [2.2.1]hept-3-yl]-3H-benzoimidazol-5-yl}-9H-fluoren-2-yl)-1H-imidazol-2-yl]-pyrrolidine-1-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester (285 mg, 59%).
1H-NMR: 300 MHz, (CD3OD-d4) δ: 8.05-7.82 (m, 9H), 5.40-5.22 (m, 2H), 4.72 (m, 1H), 4.39 (d, 1H), 4.239d, 1H), 4.17 (m, 1H), 3.91 (m, 2H), 3.62 (d, 6H), 2.98 (m, 1H), 2.58 (m, 1H), 2.37-2.18 (m, 4H), 2.18-1.92 (m, 4H), 1.80 (m, 2H), 1.09-0.85 (m, 12H). 19F-NMR: 300 MHz, (CD3OD-d4) δ: −112.88. LCMS-ESI+: calc'd for C47H52F2N8O6 862.96. Found: 863.5 (M+H+).
..................................................
SEE
WO 2010132601
WO 2013040492
WO 2013059630
WO 2013059638
.......................................
The Discovery of Ledipasvir (GS-5885), a Potent Once-Daily Oral NS5A Inhibitor for the Treatment of Hepatitis C Virus Infection
J. Med. Chem., Just Accepted Manuscript
DOI: 10.1021/jm401499g
Publication Date (Web): December 9, 2013
25 B. Synthesis of 26 and 27
25 26 27 [0186] To a flask was charged 25 (20.00 g, 0.083 mol), 4-bromo-l,2-benzenediamine (16.74 g, 0.089 mol, 1.08 equiv.), hydroxybenzotriazole (HOBt) (13.96 g, 0.091 mol, 1.1 equiv.), and l-ethyl-3-(3-dimethylaminopropyl) carbodiimide HC1 (EDC.HC1) (17.48 g, 0.091 mol, 1.1 equiv.). The flask was cooled in an ice bath, and was charged with N,N- dimethylacetamide (DMAc, 80 mL). The reaction was allowed to cool to ca. 10 °C with stirring. N-methylmorpholine (NMM) (27.34 mL, 0.249 mol, 3 equiv.) was added over 5 minutes keeping the internal temperature below 20 °C. The reaction was stirred at rt for 20 h. Upon reaction completion, the reaction mixture was added to MTBE (200 mL) and water (600 mL) in a separatory funnel and was gently shaken. The layers were allowed to separate, and the aqueous layer was removed. The aqueous layer was extracted twice with MTBE (50 mL), and the organic extracts were combined. The combined organic extracts were then extracted with water (500 mL), forming a mixture that did not separate well. The mixture was filtered over an appropriate solid support and the layers were separated. The organic phase was concentrated under vacuum, and the resulting residue was dissolved in diisopropyl ether (100 mL). The solution was cooled to ca. 5 °C with stirring. Acetic acid (5.22 mL, 0.091 mol, 1.1 equiv.) was added slowly keeping the internal temperature below 10 °C, and the resulting suspension was stirred 2 h at 5 °C. The thick suspension was then filtered, and the solid was rinsed with diisopropyl ether (100 mL), followed by heptane (100 mL). The cake was dried under vacuum to give the product as a light-beige solid as a mixture of regioisomers 26 and 27 (28.19 g, 72%, >99% AN). 1H NMR (400 MHz, DMSO) mixture of 26 & 27 (data is for the two rotamers of the major regioisomer): δ 9.25 (s, 0.5H), 9.13 (s, 0.5H), 7.08 (d, J= 8.3 Hz, 0.5H); 7.06 (d, J= 8.2 Hz, 0.5H), 6.92 (d, J= 2.2 Hz, 0.5H), 6.89 (d, J= 2.1 Hz, 0.5H), 6.71 (dd, J= 8.4, 2.2, 0.5H), 6.66 (dd, J= 8.4, 2.2, 0.5H), 5.10 (br s, 1H), 5.05 (br s, 1H), 4.15 (br s, 0.5H), 4.10 (br s, 0.5H), 3.76 (s, 1H), 2.64 (br s, 1H), 1.96- 1.88 (m, 1H), 1.77-1.67 (m, 1H), 1.67-1.19 (m, 4H), 1.41 (s, 4.5H), 1.33 (s, 4.5H). MS-ESI+: [M + H]+ calcd for Ci8H25Br03N3, 410.1, 412.1; found, 410.0, 412.0 [0187] The disclosure provides in some embodiments the use of other coupling reagents. These include but are not limited to N,N"-dicyclohexylcarbodiimide (DCC), NJV- diisopropylcarbodiimide (DIC), 6-chloro-2,4-dimethoxy-s-triazine (CDMT), O- benzotriazole-N^N^A^-tetramethyl-uronium-hexafluoro-phosphate (HBTU), and 2-(7-Aza- 1H- benzotriazole-l-yl)-l,l,3,3-tetramethyluronium hexafluorophosphate (HATU). [0188] The amine base also can be varied or omitted completely. For instance the amine is selected from tertiary amines (R3N), 2,6-lutidine, pyridine, dicyclohexylmethylamme, and N- methylmorpholine (NMM). [0189] Suitable solvent alternatives are selected from DMF, NMP, dialkyl and cyclic ethers R20, THF, 2-MeTHF, DCM, DCE, toluene, EtOAc, IP Ac, acetone, MIBK, and MEK. [0190] Suitable temperatures for the reaction range from about -20 °C to 80 °C. ...................................................
NMR PREDICT


COSY
Links
1)Link, John O.et al; The Discovery of Ledipasvir (GS-5885), a Potent Once-Daily Oral NS5A Inhibitor for the Treatment of Hepatitis C Virus Infection; Journal of Medicinal Chemistry (2013), Ahead of Print.DOI:10.1021/jm401499g
2)Ray, Adrian S. et al; Preparation of pyridazinylmethylimidazopyridine derivatives and analogs for use in the treatment of hepatitis C virus using combination chemotherapy, PCT Int. Appl., WO2013040492
3) Delaney, William E. et al ; Preparation of pyridazinylmethylimidazopyridine derivatives and analogs for use in the treatment of hepatitis C virus using combination chemotherapy, PCT Int. Appl., wo2012087596
4) Delaney, William E., IV et al; Preparation of quinoline derivatives and analogs for use in the treatment of hepatitis C virus infection in combination with ribavirin; PCT Int. Appl., wo2011156757
5) Guo, Hongyan et al; Preparation of biaryls, arylheteroaryls, heteroaryls, biarylacetylenes and related compounds end-capped with amino acid or peptide derivatives as antiviral agents; PCT Int. Appl., WO2010132601
6)Phase III (Sofosbuvir + Ledipasvir) ION-1 study: (Clinical Trial number: NCT01701401):
Title:A Phase 3, Multicenter, Randomized, Open-Label Study to Investigate the Efficacy and Safety of Sofosbuvir/Ledipasvir Fixed-Dose Combination (FDC) +/- Ribavirin for 8 Weeks and Sofosbuvir/Ledipasvir Fixed-Dose Combination (FDC) for 12 Weeks in Treatment-Naive Subjects With Chronic Genotype 1 HCV Infection
7) Phase III (Sofosbuvir + Ledipasvir) ION-2 study: (Clinical Trial number: NCT01768286)
Title:A Phase 3, Multicenter, Randomized, Open-Label Study to Investigate the Efficacy and Safety of Sofosbuvir/GS-5885 Fixed-Dose Combination ± Ribavirin for 12 and 24 Weeks in Treatment-Experienced Subjects With Chronic Genotype 1 HCV Infection
8) Phase III (Sofosbuvir + Ledipasvir) ION-3 study: (Clinical trial number: NCT01851330)
Title:A Phase 3, Multicenter, Randomized, Open-Label Study to Investigate the Efficacy and Safety of Sofosbuvir/Ledipasvir Fixed-Dose Combination (FDC) +/- Ribavirin for 8 Weeks and Sofosbuvir/Ledipasvir Fixed-Dose Combination (FDC) for 12 Weeks in Treatment-Naive Subjects With Chronic Genotype 1 HCV Infection


CLIP
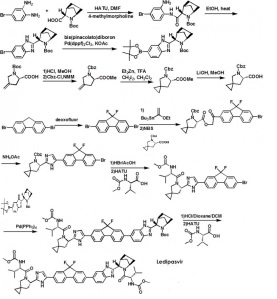
Ledipasvir (Harvoni ) Ledipasvir is a potent NS5A inhibitor that is approved for use in combination with sofosbuvir, a nucleotide inhibitor of viral polymerase, for the treatment of chronic hepatitis C virus genotype 1 infection.14,130,131 This combination was discovered and developed at Gilead Sciences and is marketed as the fixed combination with brand name of Harvoni . The synthesis of ledipasvir has been reported in the literature132 and the routes shown in Schemes 22–24 below represent the most efficient and largest scale sequence reported in the patent literature.133,134 The synthesis of the spirocyclopropane proline intermediate 136 is described in Scheme 21. Bis-iodination of cyclopropane-1,1-diyldimethanol (131) in the presence of triphenylphosphine gave diiodide 132 in 70% yield. N-Boc-glycine ethyl ester (133) was then treated with sodium hydride followed by diiodide 132 to give the protected proline analog 134 in 61% yield. Saponification of the ester followed by a classical resolution with (1S,2R)-amino-indanol gave enantomerically pure salt 135. Liberation of the free acid with 1 M HCl followed by treatment with potassium tert-butoxide provided enantiopure potassium salt 136 in high yield. The synthesis of the difluoro-fluorene Suzuki coupling intermediate 143 is described in Scheme 22. Iodination of 2-bromofluorene (137) produced aryl iodide 138 in 95% yield, which was then treated with lithium hexamethyldisilazide and N-fluorobenzenesulfonimide (NFSI) to give the difluoro intermediate 139 in 82% yield. Formation of the Grignard reagent of 139 through reaction with isopropylmagnesium chloride followed by condensation with Weinreb amide 140 gave chloroketone 141 in 71% yield. The potassium salt of the cyclopropyl proline intermediate 136 (described in Scheme 21) was coupled with 141 to give keto ester 142 in high yield. Heating 142 with ammonium acetate resulted in formation of the imidazole ring in intermediate 143 in 77% yield. The completion of the synthesis of ledipasvir is described in Scheme 23. Commercially available (1R,3S,4S)-N-Boc-2-azabicyclo [2.2.1]heptane-3-carboxylic acid (144) was coupled to 4-bromo- 1,2-benzenediamine (145) using EDC/HOBt to give a mixture ofamides 146a/146b in 72% yield. Heating mixture 146a/146b with acetic acid affected cyclization to benzimidazole 147 in 94% yield. Palladium mediated coupling of bromide 147 to bis(pinacolato)diboron gave intermediate148 which was then coupled in the same reaction vessel to bromide 143 generated in Scheme 22. This was followed by formation of the oxalate salt to give the protected central core of ledipasvir (149) in good overall yield. Removal of the amine protecting groups gave diamine 150 which was coupled to two equivalents of Moc-valine (151) via EDC/HOBt to give ledipasvir XVII in 73% yield. 19. Lobeglitazone sulfate
130. Gentile, I.; Buonomo, A. R.; Borgia, F.; Castaldo, G.; Borgia, G. Expert Opin.Invest. Drugs 2014, 23, 561.
131. Smith, M. A.; Chan, J.; Mohammad, R. A. Ann. Pharmacother. 2015, 49, 343.132. Link, J. O.; Taylor, J. G.; Xu, L.; Mitchell, M.; Guo, H.; Liu, H.; Kato, D.;Kirschberg, T.; Sun, J.; Squires, N.; Parrish, J.; Keller, T.; Yang, Z. Y.; Yang, C.;Matles, M.; Wang, Y.; Wang, K.; Cheng, G.; Tian, Y.; Mogalian, E.; Mondou, E.;Cornpropst, M.; Perry, J.; Desai, M. C. J. Med. Chem. 2014, 57, 2033.
133. Guo, H.; Kato, D.; Kirschberg, T. A.; Liu, H.; Link, J. O.; Mitchell, M. L.; Parrish, J.P.; Squires, N.; Sun, J.; Taylor, J.; Bacon, E. M.; Canales, E.; Cho, A.; Cottell, J. J.;Desai, M. C.; Halcomb, R. L.; Krygowski, E. S.; Lazerwith, S. E.; Liu, Q.;Mackman, R.; Pyun, H. J.; Saugier, J. H.; Trenkle, J. D.; Tse, W. C.; Vivian, R. W.;Schroeder, S. D.; Watkins, W. J.; Xu, L.; Yang, Z. Y.; Kellar, T.; Sheng, X.; Clarke,M. O. N. H.; Chou, C. H.; Graupe, M.; Jin, H.; McFadden, R.; Mish, M. R.;Metobo, S. E.; Phillips, B. W.; Venkataramani, C. WO Patent 2010132601A1,2010.
134. Scott, R. W.; Vitale, J. P.; Matthews, K. S.; Teresk, M. G.; Formella, A.; Evans, J.W. US Patent 2013324740A1, 2013.
135. Jin, S. M.; Park, C. Y.; Cho, Y. M.; Ku, B. J.; Ahn, C. W.; Cha, B.-S.; Min, K. W.;Sung, Y. A.; Baik, S. H.; Lee, K. W.; Yoon, K.-H.; Lee, M.-K.; Park, S. W. Diab.Obes. Metab. 2015, 17, 599.
136. Lee, H. W.; Ahn, J. B.; Kang, S. K.; Ahn, S. K.; Ha, D.-C. Org. Process Res. Dev.2007, 11, 190.
137. Lee, H. W.; Kim, B. Y.; Ahn, J. B.; Kang, S. K.; Lee, J. H.; Shin, J. S.; Ahn, S. K.; Lee,S. J.; Yoon, S. S. Eur. J. Med. Chem. 2005,
131. Smith, M. A.; Chan, J.; Mohammad, R. A. Ann. Pharmacother. 2015, 49, 343.132. Link, J. O.; Taylor, J. G.; Xu, L.; Mitchell, M.; Guo, H.; Liu, H.; Kato, D.;Kirschberg, T.; Sun, J.; Squires, N.; Parrish, J.; Keller, T.; Yang, Z. Y.; Yang, C.;Matles, M.; Wang, Y.; Wang, K.; Cheng, G.; Tian, Y.; Mogalian, E.; Mondou, E.;Cornpropst, M.; Perry, J.; Desai, M. C. J. Med. Chem. 2014, 57, 2033.
133. Guo, H.; Kato, D.; Kirschberg, T. A.; Liu, H.; Link, J. O.; Mitchell, M. L.; Parrish, J.P.; Squires, N.; Sun, J.; Taylor, J.; Bacon, E. M.; Canales, E.; Cho, A.; Cottell, J. J.;Desai, M. C.; Halcomb, R. L.; Krygowski, E. S.; Lazerwith, S. E.; Liu, Q.;Mackman, R.; Pyun, H. J.; Saugier, J. H.; Trenkle, J. D.; Tse, W. C.; Vivian, R. W.;Schroeder, S. D.; Watkins, W. J.; Xu, L.; Yang, Z. Y.; Kellar, T.; Sheng, X.; Clarke,M. O. N. H.; Chou, C. H.; Graupe, M.; Jin, H.; McFadden, R.; Mish, M. R.;Metobo, S. E.; Phillips, B. W.; Venkataramani, C. WO Patent 2010132601A1,2010.
134. Scott, R. W.; Vitale, J. P.; Matthews, K. S.; Teresk, M. G.; Formella, A.; Evans, J.W. US Patent 2013324740A1, 2013.
135. Jin, S. M.; Park, C. Y.; Cho, Y. M.; Ku, B. J.; Ahn, C. W.; Cha, B.-S.; Min, K. W.;Sung, Y. A.; Baik, S. H.; Lee, K. W.; Yoon, K.-H.; Lee, M.-K.; Park, S. W. Diab.Obes. Metab. 2015, 17, 599.
136. Lee, H. W.; Ahn, J. B.; Kang, S. K.; Ahn, S. K.; Ha, D.-C. Org. Process Res. Dev.2007, 11, 190.
137. Lee, H. W.; Kim, B. Y.; Ahn, J. B.; Kang, S. K.; Lee, J. H.; Shin, J. S.; Ahn, S. K.; Lee,S. J.; Yoon, S. S. Eur. J. Med. Chem. 2005,
THE VIEWS EXPRESSED ARE MY PERSONAL AND IN NO-WAY SUGGEST THE VIEWS OF THE PROFESSIONAL BODY OR THE COMPANY THAT I REPRESENT
References
- "Ledipasvir" (PDF). United States Adopted Name.
- "Ledipasvir-submitted-to-FDA".
- "GS-5885". Gilead Sciences.
- ELECTRON: 100% Suppression of Viral Load through 4 Weeks’ Post-treatment for Sofosbuvir + Ledipasvir (GS-5885) + Ribavirin for 12 Weeks in Treatment-naïve and -experienced Hepatitis C Virus GT 1 Patients. Gane, Edward et al. 20th Conference on Retroviruses and Opportunistic Infections. March 3–6, 2013. Abstract 41LB.
- CROI 2013: Sofosbuvir + Ledipasvir + Ribavirin Combo for HCV Produces 100% Sustained Response. Highleyman, Liz. HIVandHepatitis.com. 4 March 2013.
- "Gilead Files for U.S. Approval of Ledipasvir/Sofosbuvir Fixed-Dose Combination Tablet for Genotype 1 Hepatitis C". Gilead Sciences. 10 February 2014.
- "U.S. Food and Drug Administration Approves Gilead’s Harvoni® (Ledipasvir/Sofosbuvir), the First Once-Daily Single Tablet Regimen for the Treatment of Genotype 1 Chronic Hepatitis C". 10 October 2014. Retrieved 10 October 2014.
- Afdhal, N; Zeuzem, S; Kwo, P; Chojkier, M; Gitlin, N; Puoti, M; Romero-Gomez, M; Zarski, J. P.; Agarwal, K; Buggisch, P; Foster, G. R.; Bräu, N; Buti, M; Jacobson, I. M.; Subramanian, G. M.; Ding, X; Mo, H; Yang, J. C.; Pang, P. S.; Symonds, W. T.; McHutchison, J. G.; Muir, A. J.; Mangia, A; Marcellin, P; Ion-1, Investigators (2014). "Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection". New England Journal of Medicine 370 (20): 1889–98. doi:10.1056/NEJMoa1402454. PMID 24725239.
- http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf
- http://www.hepatitisc.uw.edu/page/treatment/drugs/ledipasvir-sofosbuvir
 |
|
| Systematic (IUPAC) name | |
|---|---|
Methyl N-[(2S)-1-[(6S)-6-[5-[9,9-Difluoro-7-[2-[(1S,2S,4R)-3-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]-3-azabicyclo[2.2.1]heptan-2-yl]-3H-benzimidazol-5-yl]fluoren-2-yl]-1H-imidazol-2-yl]-5-azaspiro[2.4]heptan-5-yl]-3-methyl-1-oxobutan-2-yl]carbamate
|
|
| Clinical data | |
| Legal status |
|
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Bioavailability | 76% |
| Protein binding | >99% |
| Metabolism | No cytochromemetabolism |
| Biological half-life | 47 hrs |
| Identifiers | |
| CAS Registry Number | 1256388-51-8 |
| ATC code | None |
| ChemSpider | 29271894 |
| ChEBI | CHEBI:85089 |
| Chemical data | |
| Formula | C49H54F2N8O6 |
| Molecular mass | 889.00 g/mol |
THE VIEWS EXPRESSED ARE MY PERSONAL AND IN NO-WAY SUGGEST THE VIEWS OF THE PROFESSIONAL BODY OR THE COMPANY THAT I REPRESENT
//////////////////////
updates............
PATENT 1
Patent application WO2010132601A1 (primary patent) discloses the base compound of ledipasvir. The application claims a general structural formula (Markush) of new amide compounds useful for treating disorders associated with HCV. This patent, if granted, serves as a blocking patent preventing competitors from making the product. The claims are very broad, using a Markush structure of antiviral agents. As per the WIPO ISR, claims 1-19 are novel and inventive. However, according to the ISR, all remaining claims (claims 20 to 173), covering a large number of compounds, lack both novelty and inventive step, due to lack of support from the patent specification and in the light of prior art. Prosecution at the USPTO Three patents have been granted in the United States: US8088368B2, claiming the base compound by general structural formula; US8273341B2 (a division of US8088368B2), claiming a method of inhibiting HCV; and US8575118B2 (a continuation of US8273341B2 and a division of US8088368B2), claiming specific amide compounds not covered in the other two related patents. The examination report of US8088368B2 reveals that the application was allowed after the applicant cancelled and amended claims on Markush substuents. The examination report of US8273341B2 reveals that the application was allowed after the applicant amended a claim 'A method of treating HCV' to 'A method of inhibiting HCV´. The examination report of US8575118B2 reveals that the application was allowed after the applicant cancelled claims already covered by the related patents, and limited claims to four specific compounds. Patent 1 has been filed in various jurisdictions: The patent has been granted by the ARIPO, in South Africa, and the United States. The patent (or a related patent) is pending in Argentina, Australia, Canada, China, as well as China, Hong Kong SAR, the EAPO, the EPO, Israel, India, Japan, New Zealand, Singapore, and Ukraine. Legal status is not available for Colombia, Ecuador, Mexico, Peru, Uruguay, and Viet Nam. 13 Litigation / Opposition on Patent 1 In December 2013, Gilead Sciences filed apatent infringement lawsuit against Abbott Laboratories and AbbVie Inc., in the United States District Court for the District of Delaware (case Number: 1:13cv02034). The case involves Gilead Sciences patents US8088368B2, US8273341B2, and US8575118B2.
PATENT 2 Patent application WO2013184698A1 is a product and process patent, claiming new crystalline solvate forms of ledipasvir useful for treating a subject suffering from HCV infection. The application also claims processes of manufacture of such amorphous and crystalline forms with specific X-ray diffraction peaks, and compositions and combinations comprising them. The application has just recently been published and no written opinion on patentability is available at this stage. As per the available information (details available in the Annex): The patent is pending at the EPO and the United States. There are no litigation or opposition procedures reported.
PATENT 3 Patent application WO2013184702A1 is a process patent, claiming processes for the preparation of ledipasvir. The disclosure also provides compounds that are synthetic intermediates to compounds of ledipasvir. The claims are moderately narrow covering crystalline and amorphous forms of ledipasvir with specific X-ray diffraction peaks. The application has just recently been published and no written opinion on patentability is available at this stage. As per the available information (details available in the Annex): The patent is pending at the EPO and the United States. There are no litigation or opposition procedures reported.
PATENT 4 Patent application WO2012087596A1 is a formulation patent, claiming various formulations comprising a combination of ledipasvir with GS-9256, or tegobuvir or with other compounds. The application also claims methods of treatment with the said combinations for reducing viral load in a person infected with HCV. 14 As per the WIPO ISR, the application is novel but not inventive in comparison to the closest prior art retrieved during the search. The combinations claimed in the instant application are not disclosed in the prior art, thus the combinations are novel. However, the prior art discloses various combinations, therefore, the problem to be solved through the invention should be new combinations with fewer side effects. Further, no experimental data of synergism has been provided to support double, triple, or quadruple combinations. Thus, according to the ISR, the instant invention cannot be regarded as inventive. As per the available information (details available in the Annex): The patent has been granted in Argentina. The patent is pending in Australia, Canada, the EPO, and the United States. Legal status is not available for Japan and Uruguay. There are no litigation or opposition procedures reported.
PATENT 5 Patent application WO2013040492A2 is a formulation and method of use patent, claiming compositions and a method of using the combination for the treatment of HCV. Drug combinations are used, and the compositions include sofosbuvir, PSI-7851 and ledipasvir. Since the application claims a group of compounds of Markush structure, it gives the claims a broad scope. As per the WIPO ISR the application is novel but lacks the inventive step in light of prior art. The invention lacks an inventive step as it would be obvious to a person skilled in the art to combine the diastereoisomer of the present invention, disclosed in the prior art, with other antiviral agents to provide an alternative HCV therapy. As per the available information (details available in the Annex): The patent is pending in Australia, Canada, the EPO, and the United States. There are no litigation or opposition procedures reported. This patent is listed in the sofosbuvir report as Patent No. 7
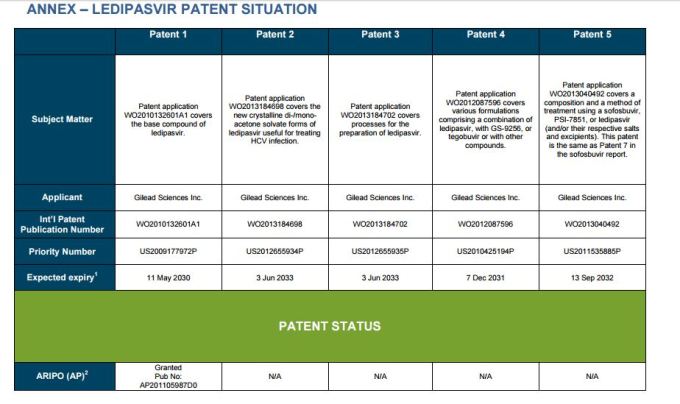
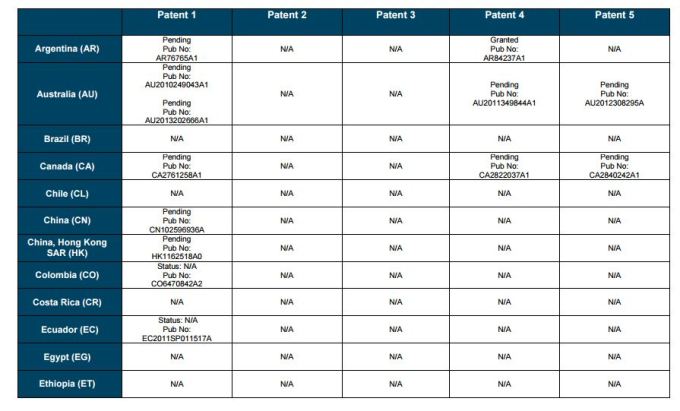
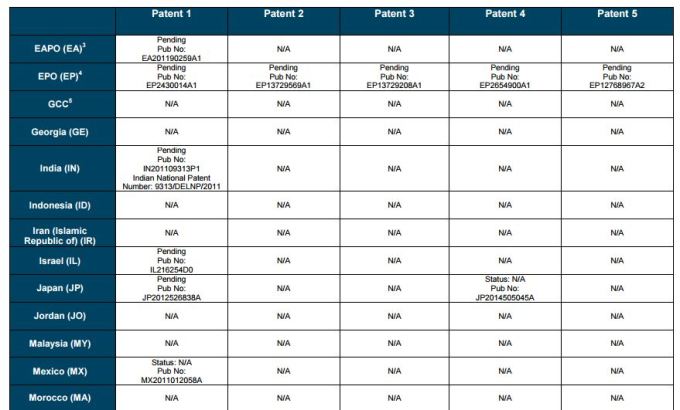
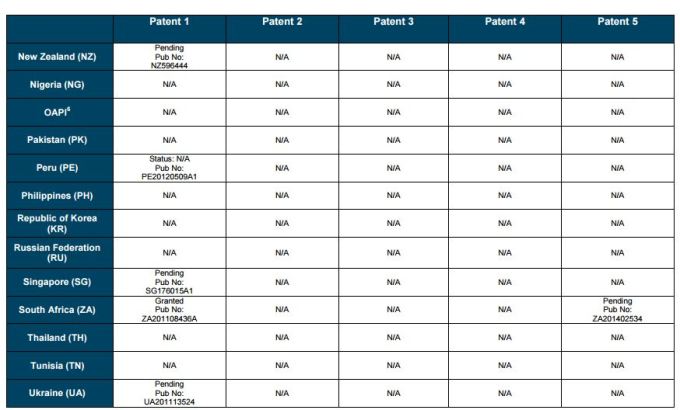
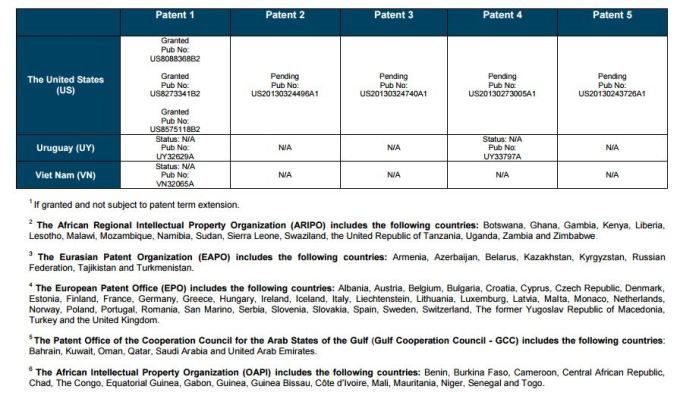
SUMMARY The search revealed patents filed with respect to ledipasvir by the Sponsor as well as a nonSponsor. The ledipasvir Sponsor patent collection comprises 5 different patents (patent families) with 47 family members published in 23 jurisdictions. The majority of these patent applications are still pending in the respective patent offices (see Patents 1 to 5 in the Annex). Patent 1 is the primary patent, claiming the base compound through a Markush claim, along with various substituents. Where granted, this patent can prevent competitors from making ledipasvir. Patents 2 and 3 claim processes to make ledipasvir and thus if granted will require competitors to design around these patents and use other production processes. The chemical product itself is not protected. Patents 4 and 5 claim combinations of different HCV drugs with ledipasvir, and their formulations. There is competition in the field by AbbVie, Inc., which filed formulation patents. Note: The search also revealed two patents that are relevant for all seven reports. Patent applications WO2013059630A1 and WO2013059638A1 inter alia claim the use of combinations of unnamed direct-acting antiviral agents for treating HCV, where the treatment does not include administration of interferon or ribavirin, and the treatment lasts between 8-12 weeks. The description and the dataset for these two patents can be found in the Working Paper on ombitasvir (Patents No 3 and 4). These patents are in litigation. Detailed information can be found in the Working Paper on sofosbuvir under Patent No 2.
update..........


WO 2016145990, Ledipasvir, New patent, SHANGHAI FOREFRONT PHARMACEUTICAL CO., LTD
(WO2016145990) METHOD OF PREPARATION FOR LEDIPASVIR AND DERIVATIVE THEREOF, AND INTERMEDIATE COMPOUND FOR PREPARATION OF LEDIPASVIR
SHANGHAI FOREFRONT PHARMCEUTICAL CO., LTD [CN/CN]; Room 1306, No.781 Cailun Road China (Shanghai) Pilot Free Trade Zone, Pudong New Area Shanghai 201203 (CN)
HUANG, Chengjun; (CN).
FU, Gang; (CN).
FU, Shaojun; (CN).
WEI, Zhewen; (CN).
LI, Wei; (CN).
ZHANG, Xixuan; (CN)
FU, Gang; (CN).
FU, Shaojun; (CN).
WEI, Zhewen; (CN).
LI, Wei; (CN).
ZHANG, Xixuan; (CN)
chinese machine translation please bear...........
Leidipawei (Ledipasvir, LDV, the structure as shown in Formula 1-LDV) was developed by Gilead hepatitis C drugs, FDA has granted LDV / SOF (Sofosbuvir) fixed dose combination drug therapy breakthrough finds that this combination therapy is expected in the short 8-week period to cure patients with genotype 1HCV, but without injections of interferon or ribavirin (ribavirin).
US20100310512 Leidipawei reported synthetic route is as follows:
2 side chain compound 1-LDV are Moc-Val, but in the compound 21 in the first to introduce Cbz-, then introduced into the left Moc-Val 13-Br in the compound by hydrolysis and condensation, and the right side chains prior to 17 -Br Boc-introduced, and then condensed by the introduction of the right hydrolyzed Moc-Val, i.e., it is not required to introducing a protecting group, then 2 times by hydrolysis, condensation of 2 times the target product. Cumbersome reaction steps, and the product raw material is expensive, tedious synthetic methods to make the product more expensive raw material costs, requires the use of more efficient ways to reduce material costs.
US2013324740 reported Leidipawei the following preparation method:
Law methodology US20100310512 efficiency in high, but still prepared Boc protected compound 24, compound 27, as well as through hydrolysis to remove the protecting group Boc, the yield is still not high, but also increase the waste emissions.
Thus, there remains the need to find simpler, more efficient Leidipawei preparation.
Route 1
Law Compound 11 first introduced in Moc-val group, Boc protection is not required, can significantly improve the synthesis efficiency and reduce waste emissions.
Route 2
Law Compound 11, Compound 3-Moc were first introduced Moc-Val, got rid of all the protection, deprotection, significantly reduced synthetic steps to improve the synthesis efficiency, production cycle reduced significantly, waste emissions significantly lower raw material costs significantly reduction, with significant industrial significance.
Route 3
Law of the compound 4-Br-Moc-Boc, the compound 5-Moc-Boc protecting group is introduced, it can reduce the effects of electron-rich N atoms of catalyst, dramatically reducing the amount of catalyst and promote the reaction, an increase of raw materials utilization. Since the catalyst and raw materials expensive, so this route can significantly reduce raw material costs. Meanwhile, the product line also reduces the defluorination impurities content.
Synthesis of Compound 1-LDV: Example 32
In three bottle was charged with compound 1'-LDV-Bz-Bz (5.25g, 4.5mmol), potassium phosphate aqueous solution (1M / L, 50mL) and tert-amyl alcohol (50 mL), warmed to 90 deg.] C, stirred for 5 hours, cooled to room temperature, ethyl acetate (100 mL). The organic phase was dried over anhydrous sodium sulfate, and concentrated to give the product (4G, yield 100%).

THANKS AND REGARD’S
DR ANTHONY MELVIN CRASTO Ph.D
DR ANTHONY MELVIN CRASTO Ph.D
GLENMARK SCIENTIST , NAVIMUMBAI, INDIA
did you feel happy, a head to toe paralysed man’s soul in action for you round the clock
need help, email or call me
MOBILE-+91 9323115463
web link
I was paralysed in dec2007, Posts dedicated to my family, my organisation Glenmark, Your readership keeps me going and brings smiles to my family














No comments:
Post a Comment