
(1R,21S,24S)-21-tert-butyl-N-((1R,2R)-1-{[(cyclopropylsulfonyl)amino]carbonyl}-2-ethylcyclopropyl)-16,16-dimethyl-3,19,22-trioxo-2,18-dioxa-4,20,23-triazatetracyclo[21.2.1.14,7.06,11]-heptacosa-6,8,10-triene-24-carboxamide
923590-37-8 cas no
| Molecular formula | C38H53N5O9S |
| Molar mass | 755.92 g mol−1 |
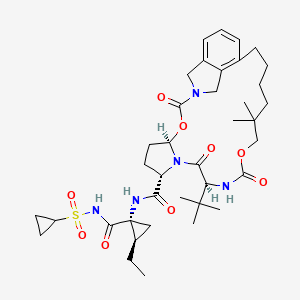
vaniprevir (MK-7009) is a macrocyclic hepatitis C virus NS3/4a protease inhibitor, is active against both the genotype 1 and genotype 2 NS3/4a protease enzymes. vaniprevir (MK-7009) has good plasma exposure and excellent liver exposure in multiple species.
Vaniprevir (MK-7009) is a macrocyclic Hepatitis C virus (HCV) NS3/4a protease inhibitor, developed by Merck & Co., which is currently in clinical testing.[1]
- McCauley JA, McIntyre CJ, Rudd MT, Nguyen KT, Romano JJ, Butcher JW, Gilbert KF, Bush KJ, Holloway MK, Swestock J, Wan BL, Carroll SS, DiMuzio JM, Graham DJ, Ludmerer SW, Mao SS, Stahlhut MW, Fandozzi CM, Trainor N, Olsen DB, Vacca JP, Liverton NJ (March 2010). "Discovery of vaniprevir (MK-7009), a macrocyclic hepatitis C virus NS3/4a protease inhibitor". J. Med. Chem. 53 (6): 2443–63.doi:10.1021/jm9015526. PMID 20163176.
Song ZJ, Tellers DM, Journet M, Kuethe JT, Lieberman D, Humphrey G, Zhang F, Peng Z, Waters MS, Zewge D, Nolting A, Zhao D, Reamer RA, Dormer PG, Belyk KM, Davies IW, Devine PN, Tschaen DM.Synthesis of vaniprevir (MK-7009): lactamization to prepare a 20-membered [corrected] macrocycle.J Org Chem. 2011 Oct 7;76(19):7804-15. Epub 2011 Aug 31.
Abstract
Development of a practical synthesis of MK-7009, a 20-membered [corrected] macrocycle, is described. A variety of ring-closing strategies were evaluated, including ring-closing metathesis, intermolecular palladium-catalyzed cross-couplings, and macrolactamization. Ring closure via macrolactamization was found to give the highest yields under relatively high reaction concentrations. Optimization of the ring formation step and the synthesis of key intermediates en route to MK-7009 are reported
Abstract
Development of a practical synthesis of MK-7009, a 20-membered [corrected] macrocycle, is described. A variety of ring-closing strategies were evaluated, including ring-closing metathesis, intermolecular palladium-catalyzed cross-couplings, and macrolactamization. Ring closure via macrolactamization was found to give the highest yields under relatively high reaction concentrations. Optimization of the ring formation step and the synthesis of key intermediates en route to MK-7009 are reported
.........................................
Kong J, * Chen C.-y, * Balsells-Padros J, Cao Y, Dunn RF, Dolman SJ, Janey J, Li H, Zacuto MJ. Merck Research Laboratory, Rahway, USA
Synthesis of the HCV Protease Inhibitor Vaniprevir (MK-7009) Using Ring-Closing Metathesis Strategy.J. Org. Chem. 2012; 77: 3820-3828
Synthesis of the HCV Protease Inhibitor Vaniprevir (MK-7009) Using Ring-Closing Metathesis Strategy.J. Org. Chem. 2012; 77: 3820-3828

2,6-Dichloro-1,4-benzoquinone was added to suppress isomerization of the allyl alkene in the isoindoline unit in C and consequent competing formation of a 19-membered ring by-product. An important contributor to the success of the RCM reaction was the high purity of crystalline B.........................................
 J. Org. Chem., 2012, 77 (8), pp 3820–3828
J. Org. Chem., 2012, 77 (8), pp 3820–3828
DOI: 10.1021/jo3001595
nmr
Synthesis of the HCV protease inhibitor vaniprevir (MK-7009) using ring-closing metathesis strategy
J Org Chem 2012, 77(8): 3820
Song, Z.G.J.; Tellers, D.M.; Journet, M.; et al.
Synthesis of vaniprevir (MK-7009): Lactamization to prepare a 22-membered macrocycle
J Org Chem 2011, 76(19): 9553
J Org Chem 2012, 77(8): 3820
Song, Z.G.J.; Tellers, D.M.; Journet, M.; et al.
Synthesis of vaniprevir (MK-7009): Lactamization to prepare a 22-membered macrocycle
J Org Chem 2011, 76(19): 9553
WO 2013106631
WO 2013101550
WO 2007015787
WO 2007015855
WO 2007015855
WO 2013066753
WO 2012082672
WO 2011025849
WO 2003099274
WO 2007016441
........................................................................................
EXAMPLE 46 (5R,7S,10S)-10-tert-Butyl-N-((1R,2R)-1-{[(cyclopropylsulfonyl)amino]carbonyl}-2-ethylcyclopropyl)-15,15-dimethyl-3,9,12-trioxo-6,7,9,10,11,12,14,15,16,17,18,19-dodecahydro-1H,5H-2,23-ethano-5,8-methano-4,13,2,8,11-benzodioxatriazacyclohenicosine-7-carboxamide (III-231)
Step 1: 8-Hydroxy-1,2,3,4-tetrahydroisoquinoline hydrobromide
A mixture of 8-methoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride [Tetrahedron Letters, 1991, 32(17), 1965.] (3.0 g 15.0 mmol) and 45 mL of 48% aqueous HBr was heated for 18 h at 120° C. The resulting brown suspension was filtered and dried to provide 8-hydroxy-1,2,3,4-tetrahydroisoquinoline hydrobromide (2.8 g, 81% yield). LRMS (ESI) m/z 150.1 [(M+H)+; calcd for C9H1NO: 150.2].
Step 2: 1-tert-Butyl 2-methyl (2S,4R)-4-{[(8-hydroxy-3,4-dihydroisoquinolin-2(1H)-yl)carbonyl]oxy}pyrrolidine-1,2-dicarboxylate:
Carbonyldiimidazole (0.176 g, 1.086 mmol) was added to a stirred, room temperature solution of DMF (5 mL) and N-Boc-trans-4-hydroxy-L-proline methyl ester (0.21 g, 0.87 mmol) and the mixture was stirred 45 min. 8-Hydroxy-1,2,3,4-tetrahydroisoquinoline (0.20 g, 0.87 mmol) and Et3N (0.18 g, 1.74 mmol) were added and the resulting solution was heated at 50° C. for 2 h. The reaction mixture was poured into aqueous saturated NH4Cl and extracted with EtOAc, dried over Na2SO4and concentrated to an oil. The residue was purified by column chromatography on silica gel (gradient elution, 10 to 80% EtOAc in hexanes) to give 1-tert-butyl 2-methyl (2S,4R)-4-{[(8-hydroxy-3,4-dihydroisoquinolin-2(1H)-yl)carbonyl]oxy}pyrrolidine-1,2-dicarboxylate (0.25 g, 0.60 mmol, 69% yield) as a colorless foam after evaporation of solvent. LRMS (ESI) m/z 321.3 [((M-Boc)+H)+; calcd for C16H21N2O5: 321.4].
Step 3: 1-tert-Butyl 2-methyl (2S,4R)-4-({[8-{[(trifluoromethyl)sulfonyl]oxy}-3,4-dihydroisoquinolin-2(1H)-yl]carbonyl}oxy)pyrrolidine-1,2-dicarboxylate
Trifluoromethanesulfonic anhydride (1.76 g, 6.24 mmol) was added to a stirred, 0° C. mixture of 1-tert-butyl 2-methyl (2S,4R)-4-{[(8-hydroxy-3,4-dihydroisoquinolin-2(1H)-yl)carbonyl]oxy}pyrrolidine-1,2-dicarboxylate (1.81 g, 4.30 mmol) and Et3N (1.31 g, 12.90 mmol) in DCM (20 mL) and stirred for 18 h. The resulting mixture was poured into saturated aqueous NaHCO3 and extracted into dichloromethane. The organic layer was washed with 10% citric acid solution, dried over Na2SO4and concentrated to red oil. The oil was purified by column chromatography on silica gel (gradient elution, 10 to 70% EtOAc in hexanes) to give a yellow oil, 1-tert-butyl 2-methyl (2S,4R)-4-({[8-{[(trifluoro methyl)sulfonyl]oxy}-3,4-dihydroisoquinolin-2(1H)-yl]carbonyl}oxy)pyrrolidine-1,2-dicarboxylate (1.65 g, 69.4% yield). LRMS (ESI) m/z 453.2 [((M-Boc)+H)+; calcd for C17H20F3N2O7S: 453.4].
Step 4: 1-tert-Butyl 2-methyl (2S,4R)-4-{[(8-vinyl-3,4-dihydroisoquinolin-2(1H)-yl)carbonyl]oxy}pyrrolidine-1,2-dicarboxylate
A solution of 1-tert-butyl 2-methyl (2S,4R)-4-({[8-{[(trifluoromethyl)sulfonyl]oxy}-3,4-dihydroisoquinolin-2(1H)-yl]carbonyl}oxy)pyrrolidine-1,2-dicarboxylate (1.74 g, 3.15 mmol), tri-n-butyl vinyl tin (1.10 g, 1.46 mmol) and lithium chloride (0.40 g, 9.45 mmol) in 25 mL DMF was purged with nitrogen for 10 min. Then bis(triphenylphosphine)palladium (II) chloride (0.22 g, 0.32 mmol) was added, and the mixture stirred at 25° C. under nitrogen for 18 h. The mixture was partitioned between EtOAc and saturated NaHCO3, the organic layer separated and washed with water then brine, dried over anhydrous sodium sulfate and concentrated to an oil. The oil was purified by column chromatography on silica gel (gradient elution, 10 to 65% EtOAc in hexanes) to give a colorless oil, 1-tert-butyl 2-methyl (2S,4R)-4-{[(8-vinyl-3,4-dihydroisoquinolin-2(1H)-yl)carbonyl]oxy}pyrrolidine-1,2-dicarboxylate (1.00 g, 74% yield). LRMS (ESI) m/z 453.2 [(M+Na)+; calcd for C23H30N2O6Na: 453.5].
Step 5: (5R,7S,10S)-10-tert-Butyl-N-((1R,2R)-1-{[(cyclopropylsulfonyl)amino]carbonyl}-2-ethylcyclopropyl)-15,15-dimethyl-3,9,12-trioxo-6,7,9,10,11,12,14,15,16,17,18,19-dodecahydro-1H,5H-2,23-ethano-5.8-methano-4,13,2,8,11-benzodioxatriazacyclohenicosine-7-carboxamide (III-231)



Synthesis of the HCV protease inhibitor Vaniprevir (MK-7009) using ring-closing metathesis strategy.
Kong J, Chen CY, Balsells-Padros J, Cao Y, Dunn RF, Dolman SJ, Janey J, Li H, Zacuto MJ.
J Org Chem. 2012 Apr 20;77(8):3820-8. doi: 10.1021/jo3001595. Epub 2012 Apr 10.
Song ZJ, Tellers DM, Journet M, Kuethe JT, Lieberman D, Humphrey G, Zhang F, Peng Z, Waters MS, Zewge D, Nolting A, Zhao D, Reamer RA, Dormer PG, Belyk KM, Davies IW, Devine PN, Tschaen DM.
J Org Chem. 2011 Oct 7;76(19):7804-15. doi: 10.1021/jo2011494. Epub 2011 Aug 31. Erratum in: J Org Chem. 2011 Nov 18;76(22):9553.
2.
McCauley JA, McIntyre CJ, Rudd MT, Nguyen KT, Romano JJ, Butcher JW, Gilbert KF, Bush KJ, Holloway MK, Swestock J, Wan BL, Carroll SS, DiMuzio JM, Graham DJ, Ludmerer SW, Mao SS, Stahlhut MW, Fandozzi CM, Trainor N, Olsen DB, Vacca JP, Liverton NJ.
J Med Chem. 2010 Mar 25;53(6):2443-63. doi: 10.1021/jm9015526.
Pompei M, Di Francesco ME, Pesci S, Koch U, Vignetti SE, Veneziano M, Pace P, Summa V.
Bioorg Med Chem Lett. 2010 Jan 1;20(1):168-74. doi: 10.1016/j.bmcl.2009.11.005. Epub 2009 Nov 10.





No comments:
Post a Comment